N-nitrosamines in 21th Century
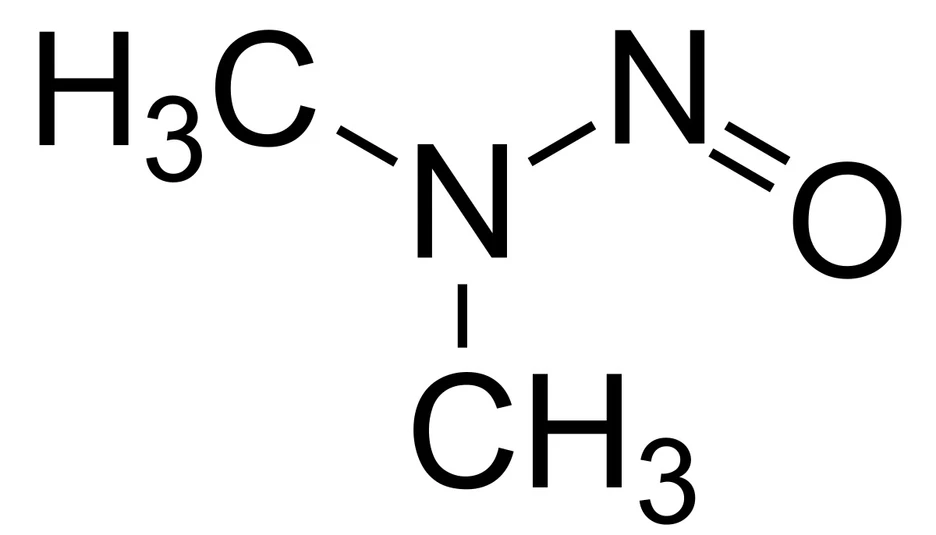
N-nitrosamines: NDMA
Carcinogenic nitrogen compounds, N-nitrosamines, were first identified in beer and malt during the 70’s of twentieth century. Their proven occurrence in beer, in context with their significant health risk, led to the development of new technological procedures, especially in malt kilning. After these technological changes, concentrations of N-nitrosamines in malts and beers were rapidly decreased and the interest of researchers in these compounds during last 15 years has consequently strongly declined even though some questions concerning these compounds have still not been answered.
1 INTRODUCTION
Nitroso compounds contain a covalently bound nitroso (-NO) group in their molecule. They can be further divided into C-, N-, Sand O-nitroso compounds. These substances are formed by direct reaction of nitrosating agents with appropriate precursors or by transnitrosation reactions, in which the nitroso groups are transferred from one molecule to another (Wainright, 1986a). N-nitroso compounds, especially N-nitrosamines, are the most important nitroso compounds in food industry. They can be characterized as N-nitrosated secondary amines with basic structure R1(R2)N-NO, where R1 a R2 are hydrocarbon radicals, aromatic rings, acyls or parts of heterocyclic rings. In terms of volatility they can be classified as volatile, less volatile and non-volatile N-nitrosamines. The group of volatile N-nitrosamines includes in particular compounds with short hydrocarbon chains or with unsubstituted heterocyclic rings, e.g. N-nitrosodimethylamine – NDMA. Less volatile N-nitrosamines are represented for example by compounds with one or two phenyl groups, e.g. N-nitrosomethylphenylamine (NMPhA) and N-nitrosoethylphenylamine (NEPhA). Conversely, non-volatile N-nitrosamines usually contain polar groups or longer alkyl chains; N-nitroso amino acids, N-nitroso heterocyclic carboxylic acids, N-nitrosoureas, N-nitrosoamides and other as yet uncharacterized compounds belong to the non-volatile group (Francis, 2000). Structures of some N-nitroso compounds are given in Fig. 1.
 Fig. 1 Structure of N-nitrosodimethylamine (NDMA), N-nitrosopyrrolidine (NPYR), N-nitrosoproline (NPRO) and N-nitrosomethylurea (NMU)
Fig. 1 Structure of N-nitrosodimethylamine (NDMA), N-nitrosopyrrolidine (NPYR), N-nitrosoproline (NPRO) and N-nitrosomethylurea (NMU)
N-nitrosamines were identified as strongly carcinogenic and mutagenic compounds (Tricker et al., 1991). Less toxic non-volatile N-nitrosamines can be transformed into more toxic volatile N-nitrosamines in a simple way. These compounds can occur in many types of food, especially after heat treatment or addition of preservative salts (Francis, 2000). They were found in grilled meat (Kocak et al., 2012), some cosmetic products (Ma et al., 2011), printing and tattoo inks (Laux et al., 2015), rubber products (Speigelhalder et al., 1982), dried fish, smoked meats, cigarette smoke and finally in malt and beer (Francis, 2000; Čulík et al., 1995).
Gas chromatography with chemiluminescence detection (GCTEA) or with mass spectrometric (GC-MS) detectors is the most common analytical method for determination of N-nitrosamines. These methods are suitable especially for volatile and less volatile N-nitrosamines while derivatization is needed for non-volatile N-nitrosamines. The method based on TEA detection is highly selective and sensitive but is has some disadvantages. Maintenance and service of TEA detectors it often complicated and this is the reason why these detectors are not common in laboratories. Another problem is a small range of standards needed for identification and quantification because the majority of structures of nitroso compounds have not yet been described (McWeeny et al., 1983). A routine and reliable analytical method for determination of non-volatile N-nitrosamines does not currently exist. Non-volatile N-nitrosamines are therefore determined together with volatile N-nitrosamines as Apparent Total N-nitroso Compounds (ATNC). ATNC are measured as the concentration of N-nitroso group in μg(N-NO)/l or kg respectively. This method is based on quantitative cleavage of N-NO bond and detection of nitric oxide or nitrosyl bromide by TEA detector. It was developed and accepted in the 70’s of twentieth century; it has not been novelized ever since and there has been no significant progress in the knowledge of ATNC compounds. Still, some studies have indicated that non-volatile N-nitrosamines represent the vast majority of total ATNC in beer (Čulík et al., 2012a; 2012b). Concentrations of ATNC in beer are usually less than 20 μg(N-NO)/l, values in the order of hundreds being usually the results of microbial contamination in some stage of brewery operations (Olšovská et al., 2014).
In malt, N-nitrosamines are formed in reactions of secondary or tertiary amino group with nitrogen oxides during kilning (Basařová et al., 2015). Precursors of NDMA in malt are especially hordenin and to a lesser extent gramin and dimethylamine (Wainwright et al., 1986a). Many N-nitrosamines do not arise primarily by nitrosation of respective amines but from either nitrosated or non-nitrosated compounds which can produce directly N-nitrosamines or their non-nitrosated analogues. For example, at higher temperatures N-nitrosoproline (NPRO) and N-nitrososarcosine (NSAR) are decarboxylated to produce N-nitrosopyrrolidine (NPYR) and NDMA (Pollock et al., 1981). Furthermore, N-nitrosated heterocyclic carboxylic acids are formed by nitrosation of condensation products of amino acids (cysteine, serine, threonine and tryptophan) and aldehydes (Francis, 2000). Compounds containing two amino groups in their structure, which are separated by four or five carbon atoms, for example lysine or some biogenic amines, can produce cyclic N-nitrosamines (Drabik-Markiewicz et al., 2011). Another precursor is creatine, which is decomposed to the amino acid sarcosine. Sarcosine can then be nitrosated (Wainright, 1986a) and thermally decarboxylated into NDMA.
N-nitrosamines can be formed also from pesticides, especially of the dinitroaniline group. In addition to some pesticides being precursors of N-nitrosamines, pesticides can be contaminated by N-nitrosamines as by-products during pesticide production (Bontoyan et al., 1979).
The source of N-nitrosamines in beer could be all feedstock or microbial contamination. Today, formation of N-nitrosamines during malting is minimalized, due to the change in kilning technology. Other beneficial factors are their high dilution factor and poor extractability into water from malt during mashing.
The situation is different with nitrate and nitrite ions, which are excellently soluble in water. Nitrites in acidic water solution form nitrous acid and further nitrogen oxides (nitrosating agents) to give N-nitroso compounds. The Czech legislation permits 0.5 mg/l as the highest concentration of nitrites in drinking water; therefore contamination from water can be excluded. The situation is worst with nitrates, which are tolerated in drinking water up to 50 mg/l and nitrate concentration in brewing water can be increased by hopping (hop is rich in nitrates). In the case of microbial contamination by nitrate reducing bacteria, nitrates can be reduced to nitrites and they can react to give N-nitrosamines (Smith, 1992).
2 LEGISLATION
According to the methodological recommendations of Czech Association of Breweries and malt houses the acceptable maximum amounts of NDMA in beer are 0.5 μg/kg, and 1.5 μg/kg for the sum of nitrosamines (including NDMA NDEA, NPYR, NPIP, NMOR and NDBA). These recommendations arise from JEFCA compendium (Joint FAO/WHO Expert Committee on Food Additives), which was part of the Czech bill no. 305/2004, which is no longer in force. Similar recommendation is applied to nitrosamines in malt, which is 1 μg/kg for NDMA and 10 μg/kg for the sum of volatile nitrosamines. For example, the recommended maximum amount for NDMA in light malt in Germany is 2.5 μg/kg (Čulík et al., 2011). No legal limits are proposed for ATNC, but values of about 20 μg(N-NO)/l are considered as satisfactory in most cases.
3 ADVANCES IN ANALYSIS OF N-NITROSAMINES
A current trend in the field of analysis of N-nitrosamines is to replace the highly selective, operationally challenging TEA detector by a versatile mass spectrometric detector. Most new analytical methods for N-nitrosamine determination have been developed for meat products, tobacco, cosmetic products and drinking or waste water, where N-nitrosamines can be formed as disinfection by-products (Nawrocki et al., 2011).
Sample preparation for determination of volatile N-nitrosamine in liquid samples (some beverages including beer) is based on solid phase extraction with polymeric carrier (Zhao et al., 2006; Jurado-Sánchez et al., 2007), active carbon or coconut charcoal (Ngongang et al., 2015; Pozzi et al., 2011) and headspace solid phase microextraction (Lona-Ramirez et al., 2015). Advanced extraction techniques for solid samples have been tested, including supercritical fluid extraction (Filho et al., 2007), microwave and ultrasonic extraction (Jurado-Sánchez et al., 2013) and solid phase microextraction with direct extraction devices which allows fast, easy and non-destructive screening of volatile N-nitrosamines and some other volatile toxic compounds in food (Ventanas et al., 2006). After the sample preparation, samples are usually separated by gas chromatography or by liquid chromatography for simultaneously determination of thermally labile and stable volatile nitrosamines and for reduction of analysis time (Zhao et al., 2006). These separation techniques are mostly coupled with mass spectrometric detection either in a tandem mass spectrometry setup (Zhao et al., 2006; Qiang et al., 2011; Ripollés et al., 2011; Boyd et al., 2011) or with high resolution mass spectrometry based on orbital ion trap (Ngongang et al., 2015; Krauss et al., 2008).
Currently, a team from the National Food Institute, Technical University of Denmark is dealing with the analysis of non-volatile N-nitrosamines in food. They developed an analytical method for simultaneous determination of volatile and non-volatile N-nitrosamines in meat products by liquid chromatography coupled with tandem mass spectrometric detection in a combination with atmospheric pressure chemical ionization and electrospray ionization (Herrmann et al., 2014). They monitored N-nitrososarcosine, N-nitrosohydroxyproline, N-nitrosoproline, N-nitroso-2-methyl-thiazolidine-4-carboxylic acid, and N-nitrosothiazolidine-4-carboxylic acid (NSAR, NHPRO, NPRO, NMTCA and NTCA) from the non-volatile group and studied the effect of some factors on resulting concentration of volatile and nonvolatile N-nitrosamines in meat products, such as the effect of temperature (Herrmann et al., 2015a) or amount of addend nitrite, erythorbic acid and ascorbyl palmitate as nitrosation inhibitors (Herrmann et al., 2015b).
The traditional but time consuming and laborious method of ATNC determination by a denitrosation mixture of hydrogen bromide and glacial acetic acid has been compared with methods using other denitrosation agents. One of these methods uses a mixture of iodine and potassium iodide in an acidic medium (Kulshrestha et al., 2010). Another method uses denitrosation mixture of cuprous chloride and hydrogen chloride. The authors of this method use one-part commercially available equipment which allows significant simplification of ATNC method and reduces problems with the relatively large apparatus used in the original method (Wang et al., 2005). However, these methods have so far been used only with meat products, soy sauce and drinking or pool water.
4 CURRENT KNOWLEDGE ABOUT THE PROPERTIES OF N-NITROSO COMPOUNDS
The properties of N-nitrosamines have not yet been fully explored, but in recent years a number of important findings have been pub lished. One of these findings is Quantitative Structure-carcinogenic Activity Relationships (QSAR) (Helguera et al., 2008). The study points out the importance of the effect of substituents on carcinogenic activity in positions R1 and R2 in symmetrical and asymmetrical N-nitrosamines with basic structure R1(R2)N-NO and of substituents in N-nitrosoureas. An enhancement of the total carcinogenic activity of N-nitrosamines has been found especially with non-substituted alkyl chains whereas unsaturated chains or hydrocarbon chains with polar groups have a negative effect on the activity. The situation is more complicated with N-nitrosoureas since the effect depends on both the type and the position of substituent in N-nitrosourea molecule. However, substances with more polar substituents have in general lower carcinogenic activity. The highest contributor to the carcinogenic activity of N-nitrosoureas is the basic central structure N-C(O)-N-NO, the substituents having a lesser influence. These conclusions are in accordance with the biotransformation theory of xenobiotics in human organism and with metabolic activation theory. These theories assume that hydrophilic substances can be directly excreted from the organism without any transformation and therefore do not pose such health risk as do lipophilic substances which have to be metabolically transformed to hydrophilic substances (Lüllmann et al., 2007); this transformation causes metabolic activation of carcinogenic properties of nitrosamines and their interaction with DNA (Kushida et al., 2000). Even though nitrosamines with polar groups exhibit little or no carcinogenic effects, they could be transnitrosated in the digestive tract into more toxic nitroso compounds (Inami et al., 2015; Inami et al., 2013).
Yurchenko et al. published an interesting study about the relationship between NDMA concentration and volume percentage of alcohol in beer (Yurchenko et al., 2005). Their data suggested that concentration of NDMA decreases with increasing volume percentage of alcohol. This dependence has been studied on different types of Estonian beers and it was observed also on light beers from different European countries. The authors explain this phenomenon by inhibitory effect of ethanol on nitrosation reactions which were described in 80’s of twentieth century (Wainright, 1986a). However, this dependence can be also hypothetically explained by assuming that more molecules of carbon dioxide are formed and exude during fermentation of beer with a higher percentage of alcohol, dragging NDMA along from the fermenting wort (oral communication, J. Čulík). At the same time the authors point out that the higher NDMA concentration in dark beers can be the consequence of higher concentration of dimethylamine in this type of beer (Yurchenko et al., 2005). This conclusion is also speculative because the major influence on NDMA concentration in dark beers is exerted by the composition of the malts used which usually contain a portion of Munich malt.
Increased formation of nitroso compounds is often due to microbial contamination. Contaminated beer has been found to contain biogenic amines that are considered to act as N-nitrosamine precursors and their formation has been studied under various experimental conditions (Drabik-Markiewicz et al., 2011). Correlation of natural logarithm of ATNC concentration and biogenic amines in beer has been also observed but no dependence was found between NDMA and ATNC (Olšovská et al., 2014; Čulík et al., 2012a). As previously described, compounds that contribute to ATNC value have not yet been characterized. However, as is clear from the basic principle of classical hydrogen bromide method of ATNC determination, compounds with N-nitroso group could not be the only substances of this group. Other compounds detectable by this method could include especially substances with C-nitroso group in the molecule. Various C-nitroso compounds have been studied, but none of them have been identified in beer or in brewery raw materials. The mechanism of C-nitrosation of polyphenols such as catechin, epicatechin or procyanidin B1, B2 and B5 in acidic medium in the presence of inorganic nitrite has been published (Hirota et al., 2015). These and related substances are natural components of hop and malt and they are extracted into the solution during brewing (Olšovská et al., 2015). C-nitroso phenols can therefore be assumed to form part of ATNC.
5 N-NITROSAMINES IN BEER AND MALT DURING THE LAST 15 YEARS
Since 1977, average NDMA concentrations in beer and malt have been gradually decreasing (Lachenmeier et al., 2007) and the occurrence of samples with a significantly higher NDMA level is unique. Higher NDMA concentrations signal a defect in technological process or the presence of microbial contamination. This situation can be illustrated by the data acquired in the Analytical Testing Laboratory
of Research Institute of Brewing and Malting. During 2001 – 2015, volatile N-nitrosamines have been determined in a total of 4115 samples of barley malt from commercial malt houses and in 1280 samples of beer from both small and large breweries, i.e. on average 274 samples of malt and 85 samples of beer per year.
The statistical evaluation included samples of Pilsner and Munich malt of Czech and foreign provenance (especially from Poland, Slovakia, Romania and Serbia) and samples of light and dark, alcoholic and non-alcoholic beer (especially from Czech Republic). During 2001–2015 the representation of Munich malts, which has often higher NDMA concentration than Pilsner malts, has been nearly constant at around 15%. Other volatile N-nitrosamines have been detected in samples only exceptionally and included mainly N-nitrosopyrrolidine and N-nitrosodiethylamine.
The mean values of NDMA concentration measured in malts and beers are shown in Fig. 2. Except for data from 2006, the values in beer are below the limit of quantification of the analytical method, which is 0.2 μg/kg. The mean values in malt are steadily around 0.8 μg/kg except for data from 2001 – 2003 and 2006.
 Fig. 2 Mean values of NDMA concentration in beers and malts in individual years
Fig. 2 Mean values of NDMA concentration in beers and malts in individual years
The percentage of samples exceeding in the respective years the limit of 0.5 μg/kg in beers and 2.5 μg/kg in malts are given in Fig. 3. These limit values have been selected on the basis of required NDMA concentration in beer, and the highest NDMA concentration recommended by German authorities in malt (see part 2, Legislation), because the stricter value recommended by Czech Association of Breweries and Malt Houses (1 μg/kg) was exceeded by 30 – 60% of malt samples. The graph shows a slight decrease of the percentage of malt samples with NDMA concentration over 2.5 μg/kg (except for data from 2001, when considerably fewer samples were analysed in comparison with other years), levelling off around 6% in last five years. A relationship between the percentage of malt samples exceeding NDMA concentration of 2.5 μg/kg and the year of determination is shown in Fig. 4. The resulting Pearson correlation coefficient is -0.81, indicating a high measure of association. The rather randomly increased NDMA concentration in beer samples (above 0.5 μg/kg) in recent years is assumed to have been caused by bacterial contamination or by using contaminated malt.
 Fig. 3 Percentage of samples exceeding 0.5 μg/kg NDMA in beer and 2.5 μg/kg in malt
Fig. 3 Percentage of samples exceeding 0.5 μg/kg NDMA in beer and 2.5 μg/kg in malt
 Fig. 4 Correlation between the percentage of malt samples with NDMA concentration above 2.5 µg/kg and the year of determination
Fig. 4 Correlation between the percentage of malt samples with NDMA concentration above 2.5 µg/kg and the year of determination
6 CONCLUSIONS
Exploration of N-nitrosamines has in the last years largely focused on such matrices as meat products, tobacco or water. These compounds, especially volatile N-nitrosamines, are still a subject of attention due to their high toxicity and, based on valid legislation, it is therefore necessary to monitor their concentration. The studies of non-volatile N-nitrosamines have reached some progress and new analytical methods developed for identification and determination of non-volatile N-nitrosamines have been successfully used for analyzing meat products. Since the 70’s of twentieth century the problem with high concentration of volatile N-nitrosamines in malts and beers has been successfully resolved but no new advances in this area have been attained apart from the substitution of TEA detection for MS detection. It should be noted that technological advances during malt production aimed to minimalize NDMA concentration may not completely prevent formation of some non-volatile N-nitrosamines, for example NPRO (Wainwright, 1986b; Smith, 1994). Toxic properties and mechanism of formation of non-volatile N-nitrosamines haven’t been sufficiently studied (apart from NPRO and NSAR), even though their presence in some foodstuffs and beverages including beer has been clearly demonstrated (Johnson et al., 1987). Low concentration of volatile N-nitrosamines should not be considered as satisfactory situation because non-volatile N-nitrosamines form a significant part of ATNC. Usual ATNC concentration in beer is below 20 μg(N-NO)/l, which is a significant decrease in comparison with the situation in the past (Massey et al., 1987). Even so, the total value of ATNC can include some portion of as yet unknown toxic non-volatile N-nitrosamines because current analytical methods cannot detect them. If such compounds have been detected in meat products, they may also occur in beer. Even though their concentrations are relatively low, intake of these compounds into the human body may pose some risk, especially with regular consumption of beer. And even though some known non-volatile N-nitrosamines haven’t been identified as carcinogenic as volatile N-nitrosamines, it turned out that they can indirectly contribute to the formation of cancer through transnitrosation in the digestive tract (Inami et al., 2013; 2015). The development is therefore needed of new analytical methods for identification and determination of compounds contributing to ATNC in beer and malt. When these compounds have been identified, it can be possible to evaluate the health risk of occasional higher values of ATNC in beers.
The Analytical Testing Laboratory of the Research Institute of Brewing and Malting, Inc., which has a long tradition of N-nitrosamine research, has launched a new research project dealing with non-volatile N-nitrosamines, their relationship to ATNC and the reasons for their production. The project will be addressed by using state-of-the-art instrumentation and completely new procedures for the preparation of solid and liquid samples.




