Determination of Rose Alcohol Composition in Extracts and Flowers via Headspace Solid-Phase Microextraction and GC-MS
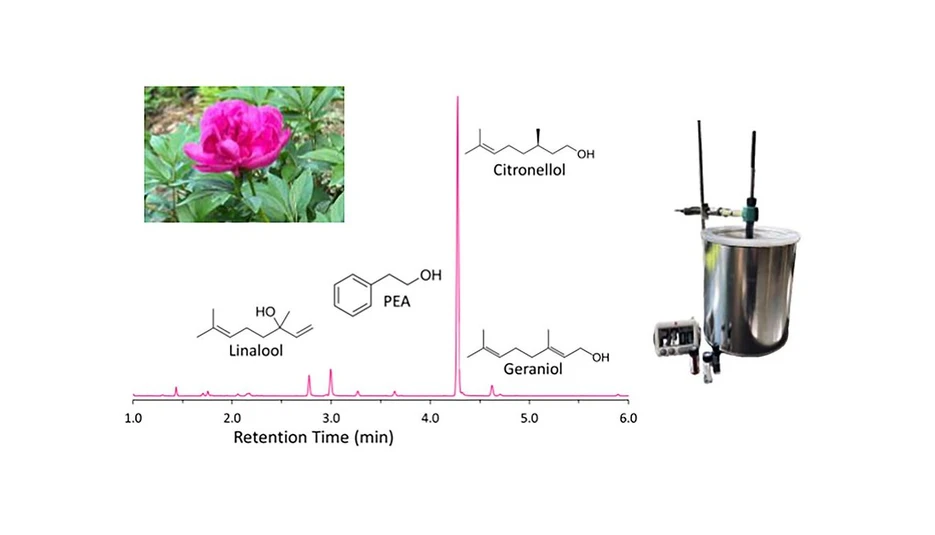
ACS Meas. Sci. Au 2025: Graphical abstract
Natural rose extracts are highly valued yet prone to adulteration, making robust analytical tools essential for verifying authenticity. In this study, GC-MS was used to characterize rose fragrances and quantify major rose alcohols, including phenyl ethyl alcohol, citronellol, and geraniol. Standard accords composed of six synthetic fragrance compounds were baseline-resolved on a nonpolar column, with calibration curves established at concentrations representative of commercial samples. Principal component analysis (PCA) further differentiated accords based on their chemical signatures.
To capture the volatile bouquet, HS-SPME was applied to fragrance strips and commercial materials, including rose absolute, rose oil, a perfume, and a peony flower. Both liquid injection and HS-SPME approaches provided complementary qualitative and quantitative insights. Overall, the results demonstrate the applicability of GC-MS and HS-SPME for evaluating rose alcohol composition in complex fragrance matrices and for identifying potential adulteration.
The original article
Determination of Rose Alcohol Composition in Extracts and Flowers via Headspace Solid-Phase Microextraction and GC-MS
Amber M. Hupp*
ACS Meas. Sci. Au 2025
https://doi.org/10.1021/acsmeasuresciau.5c00112
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
With the availability of routine synthetic methods, some fragrance houses will opt to utilize entirely synthetic accords that replicate the desired olfactory profile of rose. These accords typically include synthetic versions of the three rose alcohols in varying proportions, along with other compounds found in natural rose (e.g., eugenol, linalool, rose oxide) or those that contribute specific aromatic facets (e.g., dimethyl acetaldehyde phenyl acetate (PADMA) or cis-3-hexenal for a green note). Such accords may also incorporate natural oils or isolated molecules (isolates) from natural oils. Many of these components have been studied for their olfactory contributions to rose accords. (10)
The most commonly employed instrumental technique for determining the composition of volatile fragrance compounds is gas chromatography, typically coupled with Mass Spectrometry (GC-MS). In this technique, fragrance oils and absolutes are diluted in an organic solvent and analyzed for both qualitative identification of chemical composition and quantitative determination of concentration. GC-MS is widely used in the literature for analyzing rose oils and absolutes, and although proprietary methods are rarely published, GC-MS remains the preferred instrumental technique in major fragrance houses. (4,5,8,10,11)
As with all volatile mixtures, the headspace vapor above a liquid exhibits a different component ratio than the liquid itself, in accordance with Raoult’s Law. Variations in the vapor pressure cause certain components to be either enriched or diminished in the vapor phase. This phenomenon has prompted interest in analyzing the headspace of fragrance materials. A widely adopted method for such analysis is solid-phase microextraction (HS-SPME). (12) In this approach, a specialized fiber is exposed to the vapor phase above a liquid or solid sample, where volatile compounds adsorb onto the fiber’s surface. The fiber is then inserted into the GC-MS injection port, where the volatiles are thermally desorbed and introduced into the chromatographic column. HS-SPME has proven effective for profiling the aroma of rose alcohols in flowers and other fragrant materials. (13−15) Moreover, HS-SPME is valuable for analyzing solid samples that are otherwise difficult to assess via conventional GC-MS. Examples include fresh roses on the bush, freshly cut flowers, and commercial products such as lotions and candles. (3,9) Most published HS-SPME studies examined samples in isolation, either the oil or the headspace, the flower or the oil, or the oil versus the absolute, rather than analyzing both simultaneously. Consequently, methodologies vary across research groups. Furthermore, no published data currently address how the composition of rose alcohols may differ from the liquid oil or absolute to its corresponding headspace of the oil or absolute or between the flower and the resulting extract.
This study presents a method for analyzing both liquid and headspace rose samples applicable throughout the production process of commercial rose materials. The objectives of this experiment are (1) to develop a method for quantifying rose alcohols in liquid products (naturals and synthetics); (2) to establish a technique for identifying volatile components via headspace sampling of liquids (oils, absolutes, perfumes) and solid materials (scent strips, flowers); and (3) to investigate the influence of extraction time on the analysis of rose alcohols in the vapor phase. A unified method applicable across all material types, from the rose flower to the natural oil product to the final fragrance product represents a significant advancement in detecting adulteration and assessing rose alcohol content in situ and has not been previously documented.
Materials and Methods
Instrumentation
Separations were performed by using an Agilent 6890 gas chromatograph coupled with an Agilent 5973 mass spectrometer (Agilent Technologies, Santa Clara, TX). The GC was equipped with a nonpolar ZB-5 column (5% phenyl polydimethylsiloxane, Phenomenex, 30 m × 0.25 mm × 0.25 μm i.d.). The oven temperature was optimized for separation of the six components in the rose accord as follows: 100 to 200 °C at 10 °C/min, yielding a total run time of 10.0 min. High purity helium was used as a carrier gas at a flow rate of 0.8 L/min. Each liquid sample was manually injected (1 μL from 10 μL syringe, Hamilton Company) with a split ratio of 200:1. Each headspace sample was desorbed from the SPME fiber by using a split ratio of 50:1. The inlet and transfer line temperatures were held at 250 and 280 °C, respectively. An electron-impact ionization source was utilized with a quadrupole mass analyzer operated in full-scan mode (m/z 40 – 600) with a sampling rate of 2.6 scans/s. The mass spectrometer source and quadrupole were held at 230 and 150 °C, respectively.
Peak identification was performed using a NIST database (NIST14, Gaithersburg, MD) as well as retention time and mass spectral comparisons to external standards. The area of each peak was identified via integration using a common threshold (Enhanced Chemstation D.03.00.611, Agilent). For chemometric analysis, peak areas were normalized using the total area under the chromatogram in Microsoft Excel 2016. Normalized peak areas were mean-centered in Pirouette 4.5 (Infometrix, Bothell, WA) prior to subsequent chemometric analysis. All figures were created using the plotting feature in Microsoft Excel.
Part 2. SPME Headspace Analysis
Sampling the headspace above a natural extract or perfume is advantageous, as only the volatile components in the sample are analyzed and materials can be sampled in situ. Using SPME, the headspace above each standard and commercial sample was analyzed for percent composition and compared to direct liquid injection results. A time-based extraction study was conducted, with two time points illustrated in Figure 5 and Table 4. Within 20 min, the more volatile PEA and citronellol were readily extracted by the SPME fiber, while the least volatile components produced only small peaks. Over time, the remaining six components become more prominent. By the 2 h mark, the peak ratios more closely resembled those observed in the liquid samples (see Figure 1). This shift indicates that the ratio of PEA to the other components changes over time, as equilibrium is not yet established at the 20 min time point.
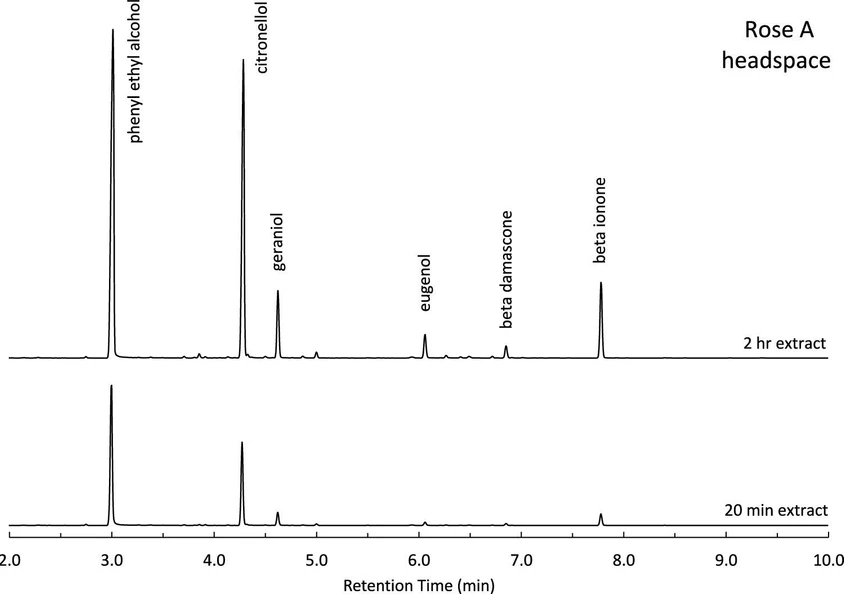 ACS Meas. Sci. Au 2025: Figure 5. Chromatography of standard rose A using SPME for 20 min and 2 h.
ACS Meas. Sci. Au 2025: Figure 5. Chromatography of standard rose A using SPME for 20 min and 2 h.
Several factors contribute to the low extraction efficiency of the least volatile components. First, differences in volatility play a key role. Larger molecules with lower vapor pressures require more time to enter the vapor phase. Second, in this study, the least volatile components were also present at low concentrations. According to Raoult’s law, components with both low concentration and low vapor pressure will be underrepresented in the headspace relative to the liquid. Third, only one type of SPME fiber was used; alternative fiber chemistries may be better suited for capturing the lower volatility compounds. Lastly, the samples were neither agitated nor heated during extraction, both known factors to accelerate equilibrium. To improve extraction of less volatile components, it is hypothesized that extended extraction time, agitation, or heating would be beneficial, assuming concentration and fiber chemistry remain constant. However, the objective of this study was not to reach equilibrium, but rather to assess whether the percent composition of rose alcohols could be determined using a rapid extraction method comparable in duration to direct liquid injection. Since rose alcohols are the most volatile of the six components in the standard, a shorter extraction time point was appropriate for this analysis.
Headspace analysis of the commercial samples was conducted using the same 20 min SPME extraction method. Chromatograms for the rose absolute and rose oil are presented in Figure 7, while for the chromatogram for the rose perfume is shown in Figure 8. As observed previously, the rose absolute consists predominately of the three rose alcohols, whereas the rose oil contains a broader array of volatile constituents. Peak intensities and areas obtained via headspace SPME differ from those observed in liquid injection, with early eluting components exhibiting enhanced signals and later eluting components being underrepresented. Nonetheless, the detection of later eluting peaks demonstrates potential for qualitative analysis of less volatile analytes, even under short nonequilibrium extraction conditions. The rose perfume sample yielded a complex chromatographic profile with numerous peaks and relatively low concentrations of the three rose alcohols, consistent with results from liquid-phase analysis. Notably, the application of HS-SPME to a fragrance strip sprayed with perfume represents a novel analytical approach, as it has not been documented in existing literature.
 ACS Meas. Sci. Au 2025: Figure 7. Chromatograms of headspace analysis by SPME of rose absolute and rose oil.
ACS Meas. Sci. Au 2025: Figure 7. Chromatograms of headspace analysis by SPME of rose absolute and rose oil.
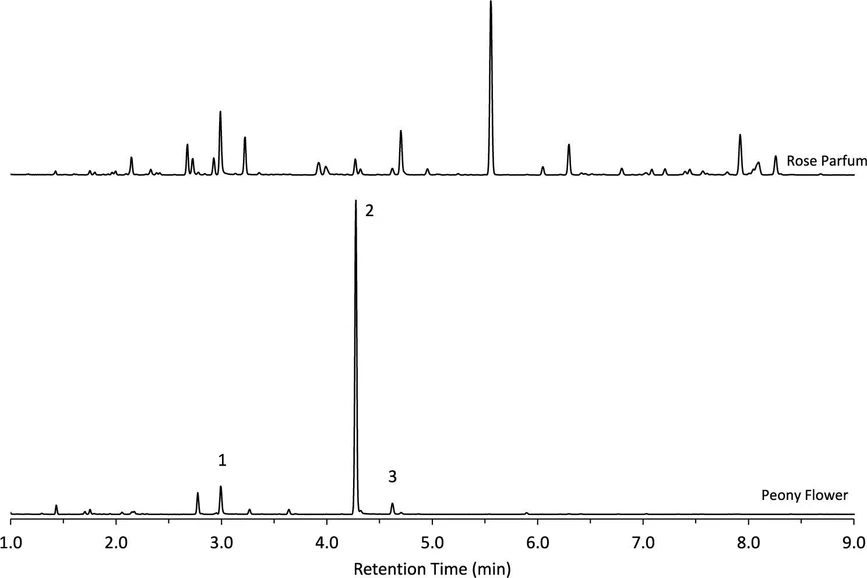 ACS Meas. Sci. Au 2025: Figure 8. Chromatogram of headspace analysis by SPME of peony flower and rose parfum. The rose alcohols are identified as 1-PEA, 2-citronellol, and 3-geraniol.
ACS Meas. Sci. Au 2025: Figure 8. Chromatogram of headspace analysis by SPME of peony flower and rose parfum. The rose alcohols are identified as 1-PEA, 2-citronellol, and 3-geraniol.
Conclusions
This study presents a robust analytical method for the quantitation of three rose alcohols, phenyl ethyl alcohol, citronellol, and geraniol, in natural rose extracts, commercial perfumes, and botanical samples. Liquid injection provided the most accurate representation of the original liquid formulations, serving as a benchmark for comparison. PCA effectively clustered rose accord standards based on the percent composition of rose alcohols and minor chemical constituents, demonstrating its utility in distinguishing sample categories and chemical profiles.
Calibration of the percent area for each rose alcohol was successfully achieved across both natural and synthetic materials without the use of an internal standard. In cases where direct liquid sampling is impractical or the sample is nonliquid, headspace analysis via HS-SPME offers a viable alternative. HS-SPME enabled rapid and effective detection of the target rose alcohols in various commercial products along with additional volatile components. While calibration accuracy decreased with increasing sample complexity, particularly in matrices containing numerous volatile compounds, the method remained capable of estimating rose alcohol percentages and identifying supplementary peaks. Notably, headspace analysis of a fresh peony flower confirmed the presence of all three rose alcohols, underscoring the potential of HS-SPME for analyzing solid botanical samples. Overall, this approach enables the determination of rose alcohol concentrations in both liquid and headspace of diverse sample types. The method offers a promising tool for monitoring compositional changes throughout the production process of rose-derived materials and may aid in the detection of adulteration in essential oils and absolutes.