VaMPIS - Validation of Measurement Procedures that Include Sampling
Eurachem: VaMPIS - Validation of Measurement Procedures that Include Sampling
Validation of analytical methods (i.e. procedures) usually excludes the primary sampling, but this is now widely recognised as the first step in the measurement procedure [1] (Fig.1). Validation of the whole measurement procedure therefore requires consideration of a performance characteristic that reflects the quality of all of the steps (including sampling and physical sample preparation). The uncertainty of the final measurement value is that key characteristic that unifies this whole measurement procedure and enables its validation, particularly when supported by performance characteristics that have an established role in the validation of an analytical procedure as a standalone activity [2]. This leaflet summarises the new Eurachem/CITAC guidance on the Validation of Measurement Procedures that Include Sampling (VaMPIS) [3]. It can be applied to the whole measurement process either simultaneously, where the sampling and analytical procedures are validated as a unified measurement procedure, or sequentially when the analytical procedure has previously been validated in isolation.
In situ and ex situ measurement
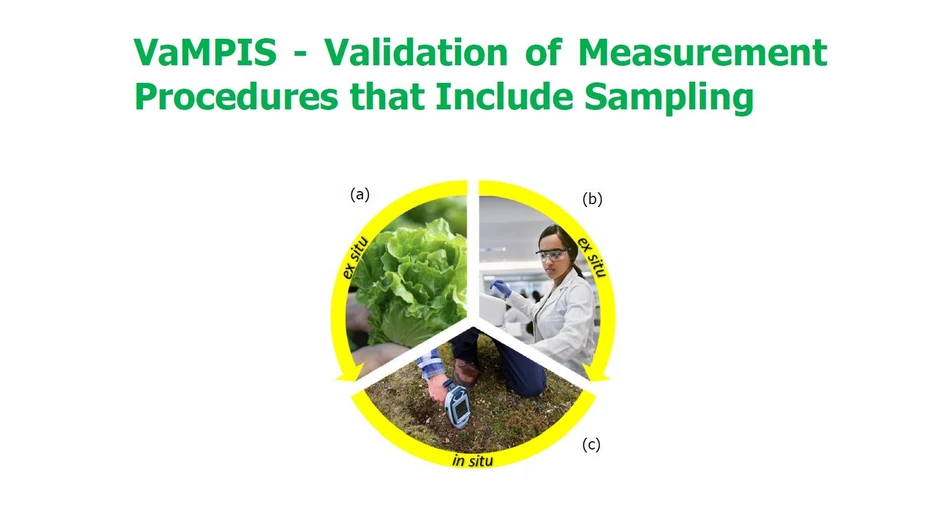
There are now many in situ measurement techniques (e.g. portable XRF in Fig. 1c), where the measurement is taken directly from the sampling target, without the need to extract a physical sample. This new mode of measurement highlights the need for an integrated approach to the definition of the measurement process so that it includes sampling. This approach is also equally applicable to the traditional mode of ex situ measurement (Fig. 1b) of a physical sample extracted from a sampling target (Fig. 1a). Both modes require the primary sampling to be included within the measurement procedure, its validation, and in the estimation of measurement uncertainty.
 Eurachem: Figure 1. Representation of the whole measurement process that requires validation. In the ex situ mode this includes the two steps of (a) primary sampling (in this case of a bay of lettuce heads) followed by (b) chemical analysis. In in situ mode this usually requires just one step (c) both sampling and analysis within the same measurement process (in this case in situ pXRF on an area of soil).
Eurachem: Figure 1. Representation of the whole measurement process that requires validation. In the ex situ mode this includes the two steps of (a) primary sampling (in this case of a bay of lettuce heads) followed by (b) chemical analysis. In in situ mode this usually requires just one step (c) both sampling and analysis within the same measurement process (in this case in situ pXRF on an area of soil).
How to apply VaMPIS
The only parameter of a measurement procedure that can effectively integrate the effects on data quality from all of the steps in the procedure (including sampling) is the measurement uncertainty. This uncertainty is stated within the final measurement result. It allows the user of that result to assess the effects of both random and systematic effects arising at every stage of the measurement procedure on decisions that are to be made using that result (e.g. compliance of a batch of material with a regulatory threshold). The VaMPIS approach therefore uses the uncertainty of the measurement value to judge the fitness for purpose (and hence validity) of the whole measurement procedure by comparing it with a ‘target’ uncertainty. It is essential that the target uncertainty should also include the contribution from sampling, and it can either be set by an external body (e.g. a regulator) or, if that is not available, by the user, using a technique such as the Optimum Uncertainty method ([3] Appendix B). Worked examples of validation are given where the whole measurement procedure is applied, either ex situ (Appendix A1: Nitrate in extracted composite samples of lettuce, Fig. 1a) or in situ (A2: Pb in soil by pXRF without removal of physical samples, Fig. 1c). There are eleven broad steps for the application of VaMPIS (Fig. 2). Measurement uncertainty is generally estimated using the Duplicate Method in which duplicate primary samples are taken from at least eight sampling targets by an independent reinterpretation of the sampling procedure. Both duplicate samples are then analysed in duplicate. The measurement uncertainty, and its main components from sampling and analysis (as repeatability), are estimated by applying ANOVA to the resultant measurement values, and also including analytical bias and sampling bias where possible [1]. The fitness for purpose of the procedure is judged by comparing the estimated measurement uncertainty against a value of target uncertainty set for that particular purpose [3].
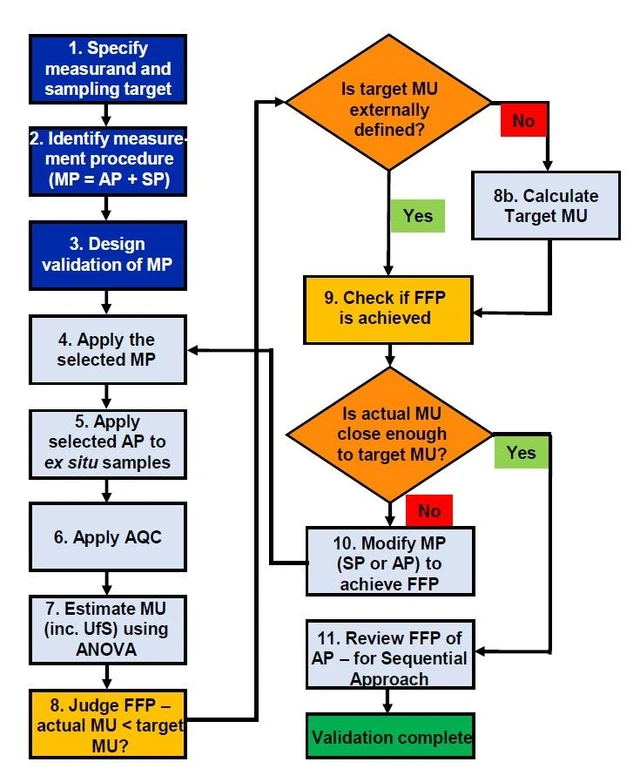 Eurachem: Figure 2. Flow chart for VaMPIS application
Eurachem: Figure 2. Flow chart for VaMPIS application
There are planning steps followed by implementation steps. The measurement uncertainty (MU) and its component arising from sampling (UfS) are usually estimated using the Duplicate Method followed by Analysis of Variance (ANOVA) [1]. The decision on whether the measurement procedure (MP) is fit for purpose (FFP) is based upon comparing the actual MU against the target MU. If not FFP, the decision on whether to improve the sampling procedure (SP) or the analytical procedure (AP) depends on their respective contributions to both the MU and the measurement cost. In the sequential approach to VaMPIS, the FFP of the AP, that has been previously validated in isolation, is checked to see if it is still FFP within this whole MP. If the MU from the analytical procedure needs to be improved, the most appropriate of the traditional performance characteristics [2] need to be modified to that end. (AQC is Analytical Quality Control.)
The VaMPIS guide [3] also includes a discussion of the role of ongoing quality control of the whole measurement procedure (integrated measurement quality control, IMQC) to ensure ongoing quality compliance after the initial validation. One major challenge for implementation that is also discussed, is to ensure effective communication and cooperation between the laboratory staff and the oftendifferent organisation that takes the samples.
Produced by the Joint Technical Group on VaMPIS with representatives from the Eurachem and AMC Sampling Uncertainty and Eurachem Method Validation Working Groups, as well as from CITAC & Nordtest. First English edition, [April 2025], www.eurachem.org
[1] Ramsey M. H., Ellison S. L. R. and Rostron P., (eds.) Eurachem/EUROLAB/CITAC/Nordtest/AMC Guide: Measurement Uncertainty Arising from Sampling: a Guide to Methods and Approaches. Eurachem (2nd ed. 2019)
[2] Cantwell H. (ed.) Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related Topics, Eurachem (3rd ed. 2025).
[3] Ramsey M.H., Rostron P.D., and Raposo F.C. (eds.) Eurachem/EUROLAB/CITAC/Nordtest/AMC, Guide: Validation of Measurement Procedures that Include Sampling, Eurachem (2024).