Rapid and Sensitive Quantification of Nano- and Microplastics in Water, Sediment, and Biological Tissue by Pyrolysis-Gas Chromatography Tandem Mass Spectrometry with Dynamic Reaction Monitoring
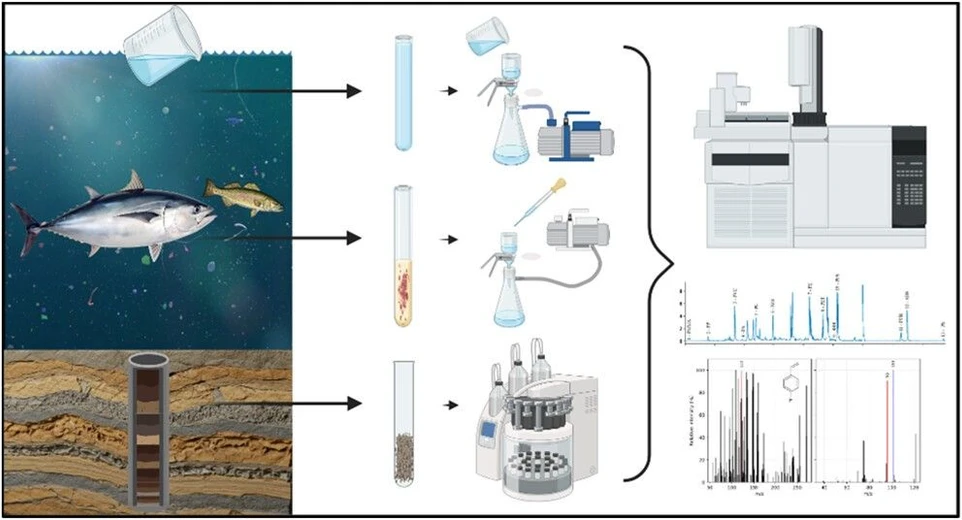
Anal. Chem. 2026, 98, 1, 633–641: Graphical abstract
A highly sensitive and selective method was developed for quantifying nano- and microplastics (NMPs) in water, sediments, and biological tissues using pyrolysis gas chromatography coupled to triple quadrupole mass spectrometry. Dynamic multiple reaction monitoring and internal standard calibration enabled nanogram-level quantification of 12 common polymers following matrix-specific sample preparation.
The method addressed matrix interferences through tailored cleanup strategies, signal enhancement with calcium carbonate, and correction of lipid-derived interferences for major polymers. Application to environmental samples from the Gulf of Mexico and the Texas coast demonstrated reliable detection of NMPs across diverse matrices, supporting high-throughput monitoring of plastic pollution.
The original article
Rapid and Sensitive Quantification of Nano- and Microplastics in Water, Sediment, and Biological Tissue by Pyrolysis-Gas Chromatography Tandem Mass Spectrometry with Dynamic Reaction Monitoring
M. Bryan Gahn*, Marcus Wharton, Asif Mortuza, David Hala, Christopher D. Marshall, Karl Kaiser*
Anal. Chem. 2026, 98, 1, 633–641
https://doi.org/10.1021/acs.analchem.5c05604
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Global plastic production has surged over the past half-century, rising from approximately 1.3 Mt in 1950 to approximately 359 Mt in 2018. (1) This increase has led to a growing concern regarding plastic pollution, particularly nano and microplastics (NMPs). Microplastics are generally defined as plastic particles with a maximum dimension of ≤ 5 mm, while nanoplastics typically range from 1 to 1000 nm in size. (2) NMPs encompass a wide variety of polymer particles that originate either from direct manufacture (e.g., microbeads and synthetic textiles) or from environmental degradation processes (e.g., photooxidation and mechanical abrasion) of larger plastic debris (i.e., macroplastics, >5 mm). (3)
NMPs are ubiquitous in the environment. They have been detected from Antarctic coast sediments to Arctic ice sheets, the summit of Mt. Everest to the depths of the Mariana Trench, and have been found in organisms across all trophic levels, including humans. (4−10) The pervasive presence of NMPs has raised significant ecological and human health concerns. NMPs can act as vectors for environmental pollutants. Hydrophobic organic contaminants (e.g., PCBs and PAHs) readily adsorb onto their surfaces, and plastics additives such as phthalates and flame retardants can leach into surrounding media (e.g., water, sediment, tissue). (11) Even without associated harmful chemicals, the physical presence of NMPs can lead to increased production of reactive oxygen species in cells, resulting in an inflammatory response. (12) Given the widespread NMP pollution and its complex ecological ramifications, there is a critical need for more robust high-throughput quantitative methods to accurately assess NMPs in diverse matrices.
Numerous analytical techniques have been employed for the detection and quantification of NMPs, which often combine traditional microscopy with advanced spectroscopic and molecular approaches. (13) These methods yield either estimates of particle counts or mass-based concentrations normalized to sample volume or weight. Prior to analysis, most methods require extensive chemical or enzymatic digestion and subsequent cleanup steps to isolate plastic particles and reduce matrix complexity. (14,15) Particle count-based methods encompass optical microscopy, laser diffraction particle size analysis, scanning electron microscopy, flow cytometry, and Fourier-transform infrared (FTIR) and Raman spectroscopy. (16) These methods provide quantitative estimates of particle abundance across the entire size spectrum of NMPs, and some can also differentiate between plastic types.
Thermal decomposition coupled with gas chromatography–mass spectrometry (GC-MS) is an increasingly utilized technique for the analysis of NMPs. (17,18) Pyrolysis-based methods thermally decompose plastic polymers in samples under oxygen-free conditions at temperatures exceeding 500 °C generating characteristic volatile fragments. These fragments are then separated by gas chromatography and detected by mass spectrometry with high sensitivity and specificity. As with other analytical techniques, pyrolysis-GC-MS (PyGC-MS) performance can be improved by incorporating sample cleanup steps prior to analysis to remove interfering organic and inorganic constituents. Matrix interference can often lead to misidentification of plastic polymer types and disrupt the pyrolysis process, ultimately compromising analytical accuracy and precision. (19) For example, lipids in biological tissues can yield similar combustion products to those of polyethylene (PE), polypropylene (PP), and nylon-66 (N-66). (20)
Improvements that enable more reliable quantification of NMPs at ultralow concentrations by PyGC-MS include optimized sample treatment methodologies that include the addition of reactants to improve pyrolysis efficiency of plastic polymers, and the application of advanced mass spectrometric methods. Quantification of NMPs at trace levels is often constrained by the minimum weight limits of analytical balances, as calibration relies on the mass of particulate standards. This limitation has been addressed by using inert fillers, such as calcium carbonate (CaCO3) or clean quartz sand that are mixed with plastic standards to produce weighable calibration standards down to the nanogram level. While single-quadrupole mass detectors are widely used due to their simplicity and low cost, they offer limited sensitivity and are less sensitive in complex matrices. (21)
The application of time-of-flight and orbitrap mass spectrometry, as well as modern triple-quadrupole mass detectors, promises improved sensitivity and specificity of NMP quantification in complex matrices. These highly selective detectors enable precise mass determination, reducing ambiguity in peak identification and enhancing the ability to differentiate plastics from natural organic matter commonly present in environmental samples. Time of flight and orbitrap mass spectrometers support untargeted screening and identification of emerging or unknown plastic-related compounds, while triple-quadrupole instruments excel in targeted quantification with high throughput and reproducibility, which are advantageous features for routine monitoring. (22−24) Compared to traditional methods such as FTIR or Raman spectroscopy, modern mass spectrometric approaches are better equipped to address the challenges posed by complex sample matrices, diverse plastic compositions, and trace-level concentrations, making them essential tools for future NMP analysis. (25,26)
Here we present a novel analytical method for the identification and quantification of NMPs in diverse environmental matrices that include marine waters, sediments, and biological tissues by using pyrolysis gas chromatography coupled with triple quadrupole mass spectrometry (PyGC-qQq-MS). Depending on the sample matrix, NMPs were concentrated through filtration, extraction, or digestion prior to analysis. Gas chromatography with qQq-MS detection using dynamic multiple reaction monitoring (DMRM) mode with an internal standard yielded significantly improved selectivity and sensitivity, enabling accurate and precise quantification of plastic polymers. Method validation demonstrated the robust performance of the method across diverse sample types, with reliable detection of NMPs at amounts of 1–126 ng and in the presence of significant matrix interference.
Experimental Section
Pyrolysis GC/MS qQq-MS Analysis
Pyrolysis gas chromatography was carried out with a Frontier Auto-Shot AS-2020E sampler and an EGA/PY-3030D multishot pyrolyzer connected to an Agilent 8890 GC and 7010B qQq-MS. Prior to pyrolysis at 600 °C, GF/F with particulates from water samples or digested tissues were spiked with PFS, which served as an internal standard. Filters were then transferred to Frontier Eco Cups and topped with 5 mg of CaCO3 and quartz wool to ensure proper packing. Redissolved sediment extracts were transferred directly into the Eco Cup LFs, dried under a gentle stream of N2, and packed with CaCO3 and quartz wool.
Results and Discussion
Pyrograms
The method was optimized to yield characteristic pyrolytic products of plastic polymers that were well resolved on a Frontier Ultra Alloy 5 column with a linear temperature program. Pyrograms of surface water, vertebrate and invertebrate tissue, and sediment samples, alongside a standard plastic mixture are shown in Figure 3. Pyrolysis temperature was optimized to ensure efficient volatilization of polymers, and specific product ions were chosen to minimize matrix influence that can generate pyrolysis products with similar mass spectra. For each polymer, precursor and product ions were selected to minimize interference from natural polymers and characteristic fragmentation reactions. For polymers that primarily undergo depolymerization (e.g., PE, PP, PS, PMMA), the selected precursor ions corresponded to characteristic oligomeric or monomeric pyrolysis products, and the quantifier ions were derived from simple bond cleavages or rearrangement reactions. For example, PS pyrolysis products included mono, di-, and trimeric units with different retention times. Mono- and dimeric fragments had retention times and mass spectra that overlapped with matrix peaks of similar mass ions, whereas the trimeric PS fragment eluted without interference from matrix components.
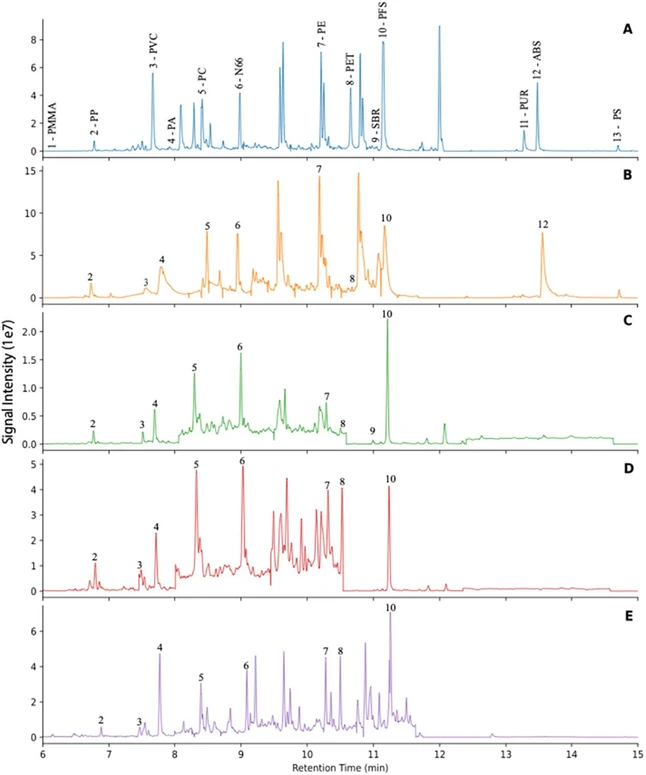 Anal. Chem. 2026, 98, 1, 633–641: Figure 3. Pyrograms of (A) Calibration standard composed of homogenized mixture containing 12 plastics of interest; (B) Surface water samples collected in Galveston Bay (2019); (C) Mullet muscle tissue collected in Galveston Bay (2021); (D) Oyster tissue collected in Galveston Bay (2021); (E) Benthic sediment collected from Matagorda Bay (2023).
Anal. Chem. 2026, 98, 1, 633–641: Figure 3. Pyrograms of (A) Calibration standard composed of homogenized mixture containing 12 plastics of interest; (B) Surface water samples collected in Galveston Bay (2019); (C) Mullet muscle tissue collected in Galveston Bay (2021); (D) Oyster tissue collected in Galveston Bay (2021); (E) Benthic sediment collected from Matagorda Bay (2023).
QqQ-MS Detection and Quantification
Optimal DMRM parameters for the detection of plastics were established using pure polymer standards (Table 1). Characteristic and abundant molecular precursor ions were identified for each plastic polymer and evaluated against natural polymers to minimize matrix interference. Fragmentation ions derived from these precursor ions were selected based on characteristic fragmentation reactions. The more abundant product ion was used for quantification, whereas the secondary ion was employed as the qualifier to monitor potential coelution with matrix compounds. Fragment losses usually corresponded to the loss of polymer subunits or dehydration reactions. Operating in DMRM mode improved sensitivity by maximizing acquisition time for each targeted analyte. Ratios of quantifier to qualifier ion (Table S2), together with retention times, ensured both compound identification and confirmation.
 Anal. Chem. 2026, 98, 1, 633–641: Table 1. Plastic Polymer Abbreviations, Relative Retention Times (RRT), Characteristic Ions, and Limit of Detection (LOD)
Anal. Chem. 2026, 98, 1, 633–641: Table 1. Plastic Polymer Abbreviations, Relative Retention Times (RRT), Characteristic Ions, and Limit of Detection (LOD)
Quantification of plastics was based on internal standard calibration using PFS as the internal standard. PFS was dissolved in toluene and added to the sample prior to pyrolysis. The use of deuterated polystyrene as an internal standard was explored; however, extensive hydrogen–deuterium exchange occurred during pyrolysis, leading to pyrolysis products with variable D/H content. (31) Similarly, attempts to use brominated polystyrene were unsuccessful, as bromine was readily lost from the polymer chain during pyrolysis, resulting in products indistinguishable from those of PS and others.
Calibration at <1 mg plastic mass was accomplished using plastic standards dispersed in CaCO3. The most consistent results were achieved with commercially available standard kits (e.g., Frontiers), which contain homogeneously distributed plastic polymers in CaCO3. Attempts to prepare homogeneous mixtures of plastic polymers in CaCO3 by ball milling finely ground plastics with CaCO3 yielded less consistent results than commercial standards.
Conclusion
This study developed and validated a robust analytical workflow for the detection and quantification of NMPs across a diverse range of environmental matrices. The method demonstrated high sample throughput, robust compensation of matrix interference, and high precision and accuracy. This was accomplished through efficient sample cleanup procedures, internal standard calibration, tandem mass spectrometry, and a correction for lipid interference in the detection of PE, PP, and N-66. The applicability of the method was demonstrated on marine surface waters, sediments and soils, and muscle tissue of fishes and oysters. Results revealed quantifiable concentrations of plastics in all sample types emphasizing the pervasive and persistent nature of plastics in the environment and their bioaccumulation potential across trophic levels. This method provides a critical tool for studying NMPs in the environment, ecological risks, and potential impacts on human health.