Influence of Polysorbate 80 on the Larvicidal and Ecotoxicological Profile of Dizygostemon riparius Essential Oil Nanoemulsion: Insights into Green Nanotechnology
J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Graphical abstract
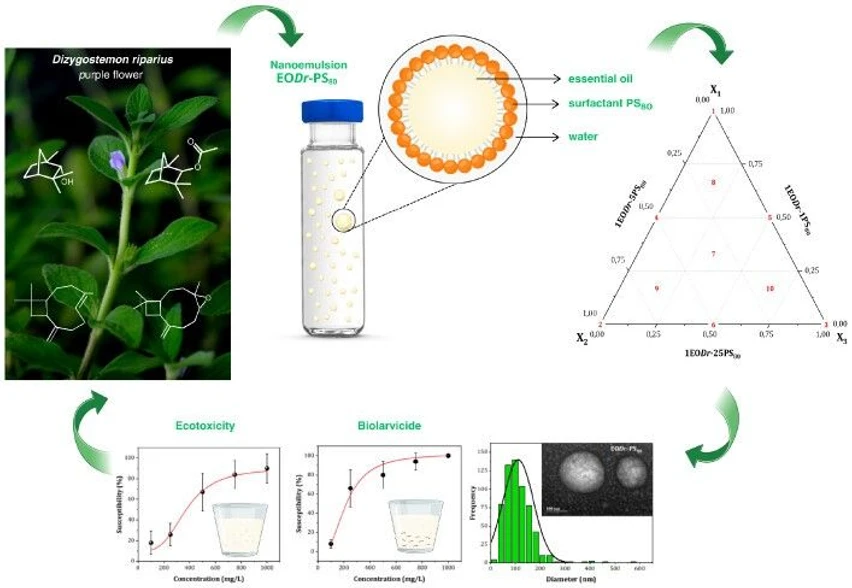
Essential-oil nanoformulations represent promising green-nanotechnology tools for mosquito control. We investigated, for the first time, the effect of Polysorbate 80 (PS80) on the larvicidal efficiency of Dizygostemon riparius essential-oil nanoemulsions (EODr) against Aedes albopictus, a vector of arboviruses such as dengue. Using a simplex-centroid design, EODr nanoemulsions were optimized and their ecotoxicological impact assessed on Artemia salina microcrustaceans.
The essential oil, obtained by hydrodistillation, contained fenchol, fenchyl acetate, caryophyllene, and caryophyllene oxide, as confirmed by GC–MS–FID and NMR analyses. Dynamic light scattering and TEM revealed spheroidal nanostructures with favorable stability (PDI, ZP). The optimized formulation displayed potent larvicidal activity and markedly lower toxicity than conventional chemical larvicides, underscoring its potential as a sustainable mosquito-control alternative.
The original article
Influence of Polysorbate 80 on the Larvicidal and Ecotoxicological Profile of Dizygostemon riparius Essential Oil Nanoemulsion: Insights into Green Nanotechnology
Clenilma M. Brandão, Djanira R. dos Santos, Lucas G. P. Silva, Mirla C. Ferreira, Joyce M. de F. Mesquita, Melissa P. Souza, Carlos A. Holanda, Renato S. Gonçalves, Emmanoel V. Costa, Georgiana E. de C. Marques, Rogério de M. Teles, Kiany S. B. Cavalcante*
J. Agric. Food Chem. 2025, 73, 31, 19327–19339
https://doi.org/10.1021/acs.jafc.5c04690
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Emerging and reemerging arboviruses represent a global public health challenge. Dengue, chikungunya, and Zika viruses are primarily transmitted by Aedes aegypti and Aedes albopictus mosquitoes. (1,2) This is a global concern, but especially in Brazil and Maranhão, where vaccination coverage is still expanding and the recurrent increase in epidemic cases raises concerns and requires urgent measures to prevent and combat the mosquito vectors. (3) The main control strategies are the use of larvicides and synthetic insecticides. (4) However, the repeated use of these synthetic compounds has contributed to the selection of resistant populations of Aedes mosquito species. (5)
In this way, the search for natural larvicides has proven to be a promising alternative strategy, with essential oils (EOs) standing out, since the complex mixture of bioactives in EOs includes classes of metabolites that give plants protection and reduce resistance against attacks by pathogens and insects. (6)
The bioactivity of EOs can be attributed to their complex mixture mainly composed of monoterpenes, sesquiterpenes, and their oxygenated derivatives. (7,8) The biological activity of EOs against Aedes mosquitoes is associated with multiple mechanisms, depending on the target action, from digestive toxicity to enzyme inhibition and toxicity to the nervous system in the larval stage. They are therefore promising, effective, and potentially environmentally safe alternatives to current synthetic larvicides. (9−11)
However, the limited availability of raw materials, low yield, high volatility, and low miscibility of EOs in aqueous media make their application in natura unfeasible. In this context, the use of surfactants is necessary, with nonionic surfactants being the most viable for use with EOs, as they reduce changes in their properties and promote the formation of micellar and/or vesicular structures with greater efficiency on a micro and/or nanoemulsion scale. (12,13)
In this context, the study focuses on the chemical composition of the EO of the plant species identified in the Cerrado biome region in the state of Maranhão, with two floral morphotypes, lilac and white, registered as Dizygostemon riparius Scatigna and Colletta (Plantaginaceae), (14) characterized the chemical profile of the EO of D. riparius (EODr) as terpenic and evaluated the larvicidal action against larvae of the A. albopictus mosquito, with this action being attributed to the EO matrix consisting of the major chemotypes, fenchyl acetate and fenchol. (15)
In the above-mentioned study, polysorbate 80 (PS80), a nonionic surfactant, was used in the composition of the emulsified system. Although PS80 is widely used in the preparation of emulsions, microemulsions and nanoemulsions, optimal concentration for formulations of mixtures with EOs is not well-defined in the literature. (16−18)
In addition to the search for emulsified systems and promising strategies to enhance the action of the bioactives present in EODr, the use of design of experiments (DOE), such as simplex centroid design (SCD), are of paramount importance to understand the interaction between the constituents of emulsified systems, in addition to increasing target-biocidal action and decreasing biological resistance. (19)
The SCD is a mathematical-statistical approach to mixture design aimed at developing and optimizing analytical responses. The adoption of this approach has proven to be methodologically effective for modeling the combined biological activities of different secondary metabolites. (20,21)
Furthermore, this type of experimental design is of great practical interest, as it involves a minimum number of experiments, the development of improved and/or innovative formulations that provide targeted responses and highlight general aspects of the interactions between independent factors. (22−24)
The article presents the formulation and characterization of EODr nanoemulsion systems and the investigation of the influence of PS80 on the larvicidal and ecotoxicological activity of emulsified EODr mixtures, using the SCD methodology, as well as their EODr-PS80 interactions.
Experimental Section
Chemical Composition: CG-FID and GC-MS
The GC-FID analysis was conducted using a Shimadzu GC-17A. The GC-MS analysis was performed using a Thermo Scientific Trace Ultra GC coupled to an ISQ MSQ. (27,28) Detailed methodology is provided in the Supporting Information.
Chemical Composition: NMR 1H and 13C
The NMR spectra of the EODr sample and the chemical standards of the main compounds, including 1D (1H, 13C, DEPT-135) and 2D (COSY, HSQC, HMBC), were recorded using a BRUKER AVANCE III HD spectrometer (Billerica, MA, USA), operating at 11.75 T (500.13 MHz for 1H NMR and 125.76 MHz for 13C NMR). Detailed methodology is provided in the Supporting Information.
Nanoemulsions Formulation
The nanoemulsions were obtained by the low-energy oil–water emulsification method (Figure 1) without the use of specialized equipment. (29) The steps of preparation of the mixtures involved some of the 12 principles of green chemistry. (30)
 J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Figure 1. General scheme for the preparation of EODr-PS80 nanoformulations.
J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Figure 1. General scheme for the preparation of EODr-PS80 nanoformulations.
Results and Discussion
Chemical Profile: GC-MS and CG-FID
The chemical profiling of the EODr matrix confirmed the plant species’ chemical composition and allowed us to associate the interactions between its constituents with the observed biological responses.
Table 1 shows the chemical composition via GC-MS and CG-FID of the EODr matrix, lilac floral morphotype, as well as the analysis of the chemical patterns (Sigma-Aldrich) of the four chemotypes in relative percentage (Figures S1–S13).
 J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Table 1. Chemical Composition of EODr, Lilac Morphotype, and Patterns of the Major Chemotypes. P = chromatogram peaks (Figures S1, S6, S8, S10, and S12); RTmin = retention time; RICalc. = calculated retention indices (on TR-5MS capillary column 30 m × 0.25 mm × 0.25 μm) according to van Den Dool and Kratz, (27) based on a homologous series of normal alkanes; RILit. = literature retention indices, Adams; (28) % = relative percentage; MF = molecular formula; TC = terpene classes.
J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Table 1. Chemical Composition of EODr, Lilac Morphotype, and Patterns of the Major Chemotypes. P = chromatogram peaks (Figures S1, S6, S8, S10, and S12); RTmin = retention time; RICalc. = calculated retention indices (on TR-5MS capillary column 30 m × 0.25 mm × 0.25 μm) according to van Den Dool and Kratz, (27) based on a homologous series of normal alkanes; RILit. = literature retention indices, Adams; (28) % = relative percentage; MF = molecular formula; TC = terpene classes.
The chemical matrix of EODr shows a terpene profile, displaying chemical compound classes of monoterpenes, oxygenated monoterpenes, sesquiterpenes and oxygenated sesquiterpenes, totaling 98.77% of the relative percentage of chemical constituents. The predominance of oxygenated monoterpenes fenchol and fenchyl acetate is significant, totaling 89.0% of the EODr matrix.
The chemical profile observed in the present study is in line with the results reported by Brandão and collaborators, (15) as well as Galvão and associates (39) who observed the terpene pattern of the EO of the same plant species, with emphasis on the chemotypes of the species, oxygenated monoterpenes, endo fenchol, 42.97%; endo fenchyl acetate, 46.03%; sesquiterpenes; (E)-caryophyllene, 4.81% and oxygenated sesquiterpenes, caryophyllene oxide, 1.94%.
The percentage variations observed in the chemical composition compared to previously published studies are due to circadian, seasonal and edaphoclimatic conditions, as the yield and chemical composition of EOs are associated with rainfall, relative humidity, solar radiation, atmospheric constitution and variations in temperature range, wind, soil and relief. (40−44)
Several studies suggest that the terpenic pattern of various chemical matrices of EOs extracted from different botanical families is correlated with high larvicidal activity against A. albopictus and A. aegypti larvae. (45,46) In this respect, studies indicate that the bioactives in the essential oil and crude extracts of the lilac morphotype of the species from the Maranhão Cerrado, D. riparius, have effective larvicidal activity, respectively, against the species A. albopictus and A. aegypti. (15,47)
Chemical Profile: 1H NMR and 13C NMR
The 1H NMR data (Table 2) provide crucial insights into the differentiation of the four major compounds in the EODr sample: fenchol, fenchyl acetate, caryophyllene, and caryophyllene oxide (Figure 3).
 J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Figure 3. Structures of the main compounds, chemical markers of the species D. riparius.
J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Figure 3. Structures of the main compounds, chemical markers of the species D. riparius.
 J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Table 2. NMR Data for 1H (125 MHz; CDCl3) of the Sample Presented as Chemical Shifts (δ)
J. Agric. Food Chem. 2025, 73, 31, 19327–19339: Table 2. NMR Data for 1H (125 MHz; CDCl3) of the Sample Presented as Chemical Shifts (δ)
Fenchol and fenchyl acetate exhibit characteristic singlets at δ 1.08 and 1.09 for C8, along with methyl signals at δ 0.98–1.03 (C9) and δ 0.86–0.77 (C10), which confirm their bicyclic structures. The presence of a doublet at δ 3.25 in fenchol and at δ 4.35 in fenchyl acetate further differentiates the hydroxyl and acetate functional groups at C2, respectively.
Caryophyllene is distinguished by its olefinic protons at δ 5.43–5.29 (H5), indicative of its bicyclo[3.3.0]octane system, whereas caryophyllene oxide exhibits shifts at δ 4.97 and 4.86 (H13), confirming the presence of an epoxide moiety. Additionally, the overlapping multiplets in the aliphatic region (δ 1.05–1.77) for all four compounds complicate signal attribution, especially due to spectral congestion from fenchol and fenchyl acetate.
However, the combined use of 13C NMR, DEPT-135, and two-dimensional NMR techniques enabled the complete assignment of all proton resonances, ensuring accurate structural characterization (Figures S14–S19).
The NMR data (Table 3) clearly distinguish the four major compounds in the EODr sample. Fenchol and fenchyl acetate exhibit similar chemical shifts, particularly at δ 49.1 and δ 48.1 (C1) and in the C3–C10 region. The key differentiating feature is the ester carbonyl signal at δ 171.6 in fenchyl acetate, absent in fenchol. Both compounds also display an oxygenated carbon at δ 85.1 and δ 86.1 (C2), indicative of a tertiary alcohol or ester functionality.




