The Chemical and Enantioselective Analysis of a New Essential Oil Produced by the Native Andean Species Aiouea dubia (Kunth) Mez from Ecuador
ACS Omega 2025, 10, 38, 44077–44086: Graphical abstract
This study provides the first description of the essential oil distilled from Aiouea dubia, a native Andean species. Chemical analyses by GC–MS and GC-FID identified major terpenes including germacrene D, γ-muurolene, limonene, δ-cadinene, cyclosativene, and (E)-β-caryophyllene.
Enantioselective GC revealed 11 chiral terpenes, with (−)-α-pinene and (−)-3-carene occurring in enantiopure form and others as scalemic mixtures. Given its high limonene and β-caryophyllene content, the oil shows potential antibacterial and anti-inflammatory properties, highlighting its promise in cosmeceutical applications.
The original article
The Chemical and Enantioselective Analysis of a New Essential Oil Produced by the Native Andean Species Aiouea dubia (Kunth) Mez from Ecuador
Katherin Fiallos, Yessenia E. Maldonado, Nixon Cumbicus, and Gianluca Gilardoni*
ACS Omega 2025, 10, 38, 44077–44086
https://doi.org/10.1021/acsomega.5c05278
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Since ancient times, plants have been used as drugs and as a source of fragrances, for both cosmetic and ritual purposes. With the introduction of the scientific method, these properties have been attributed to the presence of secondary or specialized metabolites, whose purification and structure elucidation greatly contributed to the progress of medicinal chemistry and cosmetology. Nowadays, most of the European botanical species have been phytochemically investigated, and their metabolic profiles are quite well-known. Therefore, in search for new natural products, chemists are currently focusing on the flora of so-called “megadiverse countries”, whose biodiversity includes a great fraction of all biological species around the world. (1) Among the megadiverse countries, Ecuador is included. In this territory, historical and logistical reasons avoided that most of the native and endemic botanical species could be so far studied for their metabolic content. (2,3) For these reasons, our group has been investigating the Ecuadorian biodiversity for more than 20 years, in search for new natural molecules, biologically active compounds, and unprecedented essential oils (EOs). (4−9) In this context, some of the authors recently described a volatile fraction from Aiouea montana (Sw.) R. Rohde, whose composition was dominated by a peculiar, sulphurated compound, responsible for the strong unpleasant smell of the leaves. (10) On the other hand, the leaves of the less common Aiouea dubia did not present a similar smell but a pleasant, quite sweet fragrance. From the botanical point of view, the genus Aiouea Aubl. is currently considered, by some botanists, the correct classification for all the Neotropical taxa, that were previously included into the genera Phoebe and Cinnamomum. (11) The taxon Aiouea is therefore native of America, and it includes 73 species with an accepted name, whereas the reported synonyms are more than 200. (12) For what concerns A. dubia, this species is a shrub, treelet or tree, native of the Andes, and diffused from Venezuela to Peru. (12−14) In Ecuador, this plant grows in the range between 2000 and 3000 m above the sea level, being described in the provinces of Loja and Zamora-Chinchipe. (14) Some synonyms are also reported for A. dubia, such as Aiouea granatensis Mez, Aiouea jelskii Mez, Aiouea tambillensis Mez, Aiouea truxillensis Kosterm, Cryptocarya dubia Kunth, Endocarpa corymbosa Raf., Laurus hypericifolia Willd. ex Nees, and Persea hypericifolia Nees. So far, to the best of the authors’ knowledge, only the EOs from Aiouea costaricensis, Aiouea maguireana, and A. montana have been investigated within this genus, whereas no literature has been found describing the chemical and the enantiomeric profiles of any volatile fraction from A. dubia or its synonyms. (10,15,16) With these premises, the species A. dubia (Kunth) Mez (Lauraceae) was selected to be analyzed for the chemical and enantiomeric compositions of its EO. Main purposes of the present study were contributing to the preservation of the Ecuadorian biodiversity through the phytochemical knowledge and identifying potential sources of new natural products, suitable to be the object of bioeconomic applications.
Methods
Qualitative (GC–MS) Chemical Analyses
The qualitative chemical composition of A. dubia EO was carried out through gas chromatography, using mass spectrometry as a detection technique (GC–MS). The GC instrument was a gas chromatograph model Trace 1310, purchased from Thermo Fisher Scientific (Waltham, MA, USA). The GC was coupled with a single quadrupole mass spectrometer (MS) model ISQ 7000, purchased from the same provider. The qualitative analyses were conducted with two capillary columns, based on stationary phases of different polarity: 5%-phenyl-methyl-polysiloxane (TR-5 ms, nonpolar) and polyethylene glycol (TR-Wax, polar), purchased form Thermo Fisher Scientific (Waltham, MA, USA). Both columns were characterized by the following dimensions: 30 m in length, internal diameter of 0.25 mm, and film thickness of 0.25 μm. The thermal program was 50 °C for 10 min, followed by an initial gradient of 2 °C/min up to 170 °C, then a second gradient of 10 °C/min up to 230 °C, which was finally maintained for 20 min. All the injections were conducted in split mode (40:1), with helium as a carrier gas, provided by Indura s.a. (Guayaquil, Ecuador), and maintained at the constant flow of 1 mL/min. Injector and transfer line were set at the temperature of 250 °C. The mass spectrometer detector was equipped with an electron ionization source, set at the ionization energy of 70 eV. The detector was operated in SCAN mode, detecting ions within the mass range of 40–400 amu. Both ion source and quadrupole temperatures were 250 °C. All the EO constituents were identified by comparison of each MS spectrum and linear retention index (LRI) with data from literature. The LRIs were calculated according to Van den Dool and Kratz, (67) based on a series of homologous n-alkanes in the range C9–C24. The alkanes were provided by Merck (Sigma-Aldrich, St. Louis, MO, USA).
Quantitative (GC-FID) Chemical Analyses
The quantitative chemical analyses were conducted with the same GC instrument and the same columns described for the qualitative ones, but with a flame ionization detector (FID), set at 280 °C, instead of the mass spectrometer. Carrier gas, constant flow, injection temperature and mode, and oven thermal program were the same as the qualitative analyses. All the detected compounds were quantified calculating the relative response factor (RRF) of each constituent according to the respective combustion enthalpy, as described by Chaintreau. (68,69) The integration areas, corrected by the RRFs, were applied to two six-point calibration curves (one for each column), that were traced using n-nonane as internal standard and isopropyl caproate as calibration standard. The internal standard was provided by Merck (Sigma-Aldrich, St. Louis, MO, USA), whereas the calibration standard was synthesized in the authors’ laboratory and purified to 98.8% (GC-FID purity). Both curves produced coefficients of determination R2 > 0.998, with standard dilutions prepared as previously described in literature. (70)
Results and Discussion
Chemical Analysis
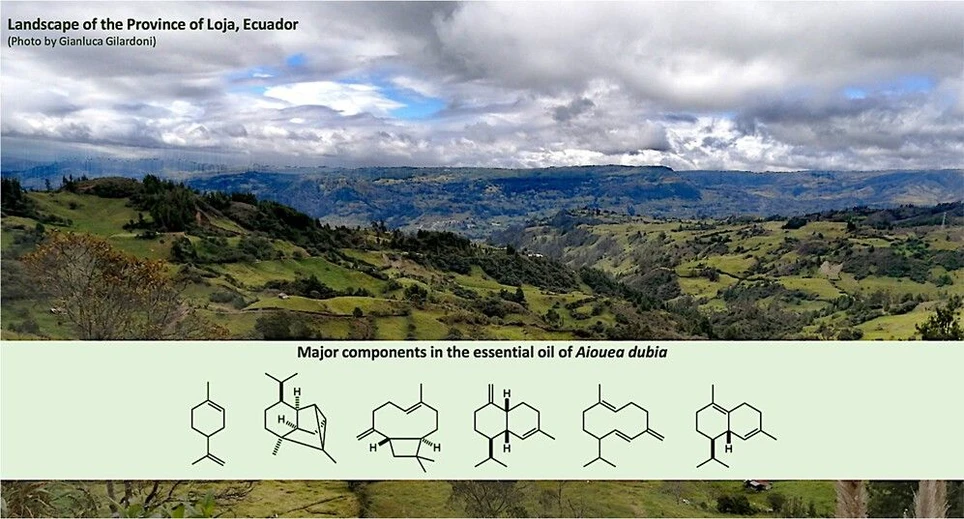
The dry leaves of A. dubia afforded, after preparative steam-distillation, a clear pale-yellow oil, characterized by a sweet light fragrance. The distillation yield, by weight, was 0.3%. A total of 69 compounds were detected and quantified on at least one of two chromatographic columns. The columns were coated with different stationary phases, being 5%-phenyl-methyl-polysiloxane a nonpolar phase and polyethylene glycol a polar phase. The great difference in polarity among these two phases ensured that chromatographic properties were also different enough to obtain a good separation, for almost all compounds, on at least one column. Furthermore, different chromatographic properties produced different linear retention indices for a same compound, ensuring a more reliable identification through the coincide of both linear retention indices with data from literature. Finally, these specific stationary phases are the most used in EO analysis, ensuring that a lot of literature is available as a source of reference linear retention indices. The total mass of the quantified components corresponded to 85.1%–82.8% of the whole oil mass, expressed as the sum of all quantified compounds on the nonpolar and polar column, respectively. This volatile fraction was dominated by sesquiterpenes and sesquiterpenoids, corresponding together to 65.5%–61.4% of the total amount. Major components of the EO (≥3.0% on at least one column) were germacrene D (12.2%–11.7%, 45), γ-muurolene (7.6%–7.2%, 44), limonene (6.8%–6.2%, 14), δ-cadinene (6.4%–5.9%, 51), cyclosativene (6.0%–5.5%, 32), and (E)-β-caryophyllene (4.4%–4.0%, 36). The chemical structures of the main EO components are represented in Figure 1, whereas the gas chromatographic (GC) profiles, obtained on a nonpolar and a polar stationary phase, are respectively represented in Figure 2 and Figure 3. The complete qualitative and quantitative analyses are reported in Table 1.
 ACS Omega 2025, 10, 38, 44077–44086: Figure 1. Major components (≥3.0% on at least one column) of A. dubia leaf EO. The numbers refer to Table 1: limonene (14), cyclosativene (32), (E)-β-caryophyllene (36), γ-muurolene (44), germacrene D (45), and δ-cadinene (51).
ACS Omega 2025, 10, 38, 44077–44086: Figure 1. Major components (≥3.0% on at least one column) of A. dubia leaf EO. The numbers refer to Table 1: limonene (14), cyclosativene (32), (E)-β-caryophyllene (36), γ-muurolene (44), germacrene D (45), and δ-cadinene (51).
 ACS Omega 2025, 10, 38, 44077–44086: Figure 2. GC–MS profile of A. dubia EO on a 5%-phenyl-methyl-polysiloxane stationary phase. The peak numbers refer to major compounds (≥3.0% on at least one column) in Table 1.
ACS Omega 2025, 10, 38, 44077–44086: Figure 2. GC–MS profile of A. dubia EO on a 5%-phenyl-methyl-polysiloxane stationary phase. The peak numbers refer to major compounds (≥3.0% on at least one column) in Table 1.
 ACS Omega 2025, 10, 38, 44077–44086: Figure 3. GC–MS profile of A. dubia EO on a polyethylene glycol stationary phase. The peak numbers refer to major compounds (≥3.0% on at least one column) in Table 1.
ACS Omega 2025, 10, 38, 44077–44086: Figure 3. GC–MS profile of A. dubia EO on a polyethylene glycol stationary phase. The peak numbers refer to major compounds (≥3.0% on at least one column) in Table 1.
Conclusions
The steam-distilled dry leaves of A. dubia produced an EO, with the non-negligible yield of 0.3% by weight. This volatile fraction was characterized by a pleasant, sweet smell, similar to the one of other sesquiterpene-based oils. Being dominated by germacrene D and due to the high enantiomeric excess of its levorotatory enantiomer, this EO could act as an attractant for the insects of the genus Heliotis. Despite A. dubia currently is an only wild species, its leaf EO could be considered commercially promising, due to its high distillation yield. Cosmeceutical science could be the main application field for this volatile fraction. In fact, the high content of limonene and (E)-β-caryophyllene suggested that A. dubia EO could present antibacterial and anti-inflammatory properties, like other oils of similar composition. Future research should experimentally investigate the biological activities of this EO, with a special focus on the two main properties deduced since literature. In fact, for cosmeceutical applications, antibacterial and anti-inflammatory capacities are possibly more interesting than other biological activities, whose actions imply a systemic mechanism instead of a topic effect.