QuEChERS Combined with Low-Temperature Partitioning and GC–MS as an Analytical Strategy for the Determination of Multiclass Pesticide Residues in Cocoa Beans
ACS Omega 2025, 10, 51, 63464–63473: Graphical abstract
This study presents an analytical strategy combining QuEChERS extraction with low-temperature partitioning (LTP) for the determination of multiclass pesticide residues in cocoa beans by GC–MS. Extraction conditions were optimized using a fractional factorial design, resulting in a simple and efficient workflow suitable for complex cocoa matrices.
The validated method showed satisfactory limits of detection and quantification, recoveries within 70–120%, and good overall performance. Application to cocoa samples from Brazil detected permethrin in several samples, demonstrating that the QuEChERS–LTP approach is an effective and reliable cleanup strategy for routine pesticide residue analysis in cocoa.
The original article
QuEChERS Combined with Low-Temperature Partitioning and GC–MS as an Analytical Strategy for the Determination of Multiclass Pesticide Residues in Cocoa Beans
Priscilla M. de Freitas Machado, Madson M. Nascimento, Paulo R. R. Mesquita, Manuela B. Nascimento, Lilian Lefol N. Guarieiro, Gisele O. da Rocha, Jailson B. de Andrade, Raildo M. de Jesus*
ACS Omega 2025, 10, 51, 63464–63473
https://doi.org/10.1021/acsomega.5c10360
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Cocoa is best known as the primary raw material for chocolate but is also processed into products such as cocoa butter, biscuits, cocoa honey, and cocoa powder. Cocoa powder is derived from roasted, pressed, and milled nibs, containing on average 20% (w/w) lipids on a dry basis and less than 9% moisture. (3,4)
According to the Brazilian Health Regulatory Agency (ANVISA), pesticides such as atrazine, bifenthrin, cyproconazole, metalaxyl, tebuconazole, trifloxystrobin, and permethrin are approved for use in Brazil. (13) However, among these, only metalaxyl, trifloxystrobin, and tebuconazole are authorized by the European Union. (14) Thus, investigating these contaminants in cocoa samples is of utmost importance, as Brazil is one of the largest cocoa exporters, supplying cocoa commodities to South America, the United States, and the European Union. (2) Furthermore, Organochlorine pesticides, in particular, belong to the class of persistent organic pollutants (POPs). Although banned in many countries, they continue to be used in some developing nations because of their chemical stability, resistance to degradation, volatility, and high lipophilicity, which promote bioaccumulation and neurotoxicity. (15) Epidemiological evidence also associates their exposure with Parkinson’s disease, certain cancers, diabetes, and endometriosis. (16)
Residues of pesticides in cocoa have been reported in various studies. (17) Analyzing pesticide residues in cocoa presents analytical challenges due to the matrix’s complexity, comprising high lipid content, fatty acids and esters, sugars, polyphenols, and caffeine. These components can interfere with extraction efficiency and contaminate analytical instrumentation. (18) Therefore, efficient sample preparation methods incorporating effective cleanup steps are essential for accurate residue determination.
Several techniques have been employed for multiresidue pesticide analysis in cocoa. For instance, Idowu et al. (19) quantified 14 organochlorines using Soxhlet extraction and silica/Na2SO4 cleanup, with GC ECD detection. Okoffo et al. (20) determined 13 organophosphates and 9 pyrethroids employing SPE cartridges (Envi-carb/LC-NH2 and Bond Elute C18), acetonitrile extraction, and GC PFPD or GC ECD quantification. Yusiasih et al. (21) applied dispersive SPE with PSA, Florisil, and MgSO4 for pyrethroids, followed by GC ECD and GC–MS.
Therefore, this study aimed to evaluate the effectiveness of a QuEChERS–LTP analytical procedure for the determination of multiclass pesticide residues in cocoa beans. The factors that affect the QuEChERS–LTP procedure efficiency were optimized. After validation, the procedure was employed to investigate the occurrence of 15 multiclass pesticides in cocoa samples.
Experimental Section
Instrumental Apparatus
Pesticide separation and identification were performed on a Shimadzu GC-MS-QP2010SE system (Kyoto, Japan) equipped with an AOC-20i autosampler. Separation was achieved using an Agilent DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm) with a stationary phase of 5% phenyl/95% dimethylpolysiloxane. The instrumental conditions were based on Nascimento et al. (49) and they are summarized in Table 4.
For quantification, the base peak was used, while two additional ions were monitored for confirmation (Table S2) in accordance with SANTE guidelines. (50) A GC–MS/SIM chromatogram illustrating the separation of all target pesticides is presented in Figure 5.
![ACS Omega 2025, 10, 51, 63464–63473: Figure 5. Extracted ion chromatogram (EIC) in SIM mode of the target pesticides obtained from the injection of a mixed standard solution at 100 μg L–1. Analytes are listed in order of elution: [1] Molinate (6.18 min); [2] α-HCH (8.15 min); [3] atrazine-d5 (8.69 min); [4] atrazine (8.75 min); [5] diazinon (9.31 min); [6] disulfoton (9.67 min); [7] dimethachlor (10.60 min); [8] metalaxyl (11.25 min); [9] p,p′-DDE (15.82 min); [10] cyproconazole I (16.50 min); [11] cyproconazole II (16.56 min); [12] p,p′-DDD (17.29 min); [13] ethion (17.34 min); [14] trifloxystrobin (18.43 min); [15] tebuconazole (19.13 min); [16] bifenthrin (20.47 min); [17] permethrin I (23.92 min); [18] permethrin II (24.18 min).](https://gcms.labrulez.com/labrulez-bucket-strapi-h3hsga3/ACS_Omega_2025_10_51_63464_63473_Figure_5_Extracted_ion_chromatogram_EIC_in_SIM_mode_of_the_target_pesticides_f2461c14f4_l.webp) ACS Omega 2025, 10, 51, 63464–63473: Figure 5. Extracted ion chromatogram (EIC) in SIM mode of the target pesticides obtained from the injection of a mixed standard solution at 100 μg L–1. Analytes are listed in order of elution: [1] Molinate (6.18 min); [2] α-HCH (8.15 min); [3] atrazine-d5 (8.69 min); [4] atrazine (8.75 min); [5] diazinon (9.31 min); [6] disulfoton (9.67 min); [7] dimethachlor (10.60 min); [8] metalaxyl (11.25 min); [9] p,p′-DDE (15.82 min); [10] cyproconazole I (16.50 min); [11] cyproconazole II (16.56 min); [12] p,p′-DDD (17.29 min); [13] ethion (17.34 min); [14] trifloxystrobin (18.43 min); [15] tebuconazole (19.13 min); [16] bifenthrin (20.47 min); [17] permethrin I (23.92 min); [18] permethrin II (24.18 min).
ACS Omega 2025, 10, 51, 63464–63473: Figure 5. Extracted ion chromatogram (EIC) in SIM mode of the target pesticides obtained from the injection of a mixed standard solution at 100 μg L–1. Analytes are listed in order of elution: [1] Molinate (6.18 min); [2] α-HCH (8.15 min); [3] atrazine-d5 (8.69 min); [4] atrazine (8.75 min); [5] diazinon (9.31 min); [6] disulfoton (9.67 min); [7] dimethachlor (10.60 min); [8] metalaxyl (11.25 min); [9] p,p′-DDE (15.82 min); [10] cyproconazole I (16.50 min); [11] cyproconazole II (16.56 min); [12] p,p′-DDD (17.29 min); [13] ethion (17.34 min); [14] trifloxystrobin (18.43 min); [15] tebuconazole (19.13 min); [16] bifenthrin (20.47 min); [17] permethrin I (23.92 min); [18] permethrin II (24.18 min).
Application of the Proposed Procedure to Real Samples
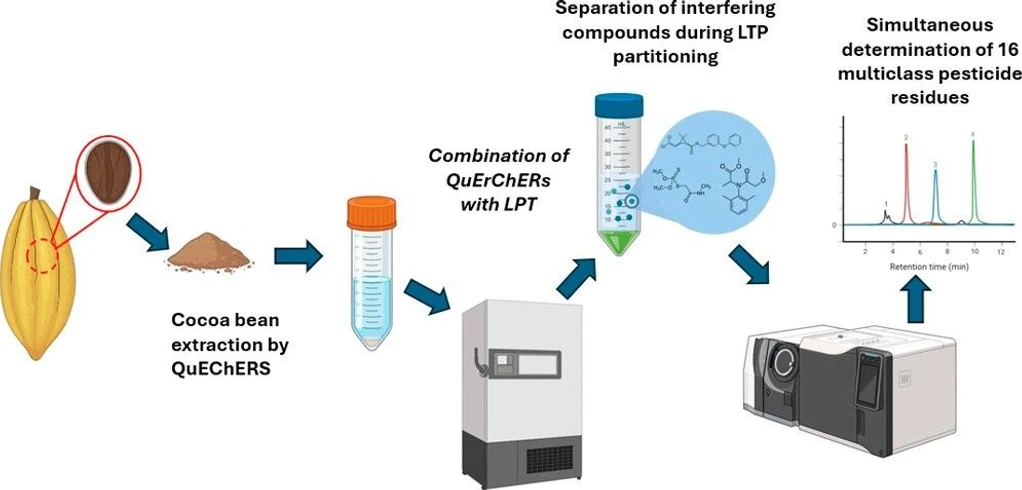
In a 50 mL Falcon tube, 2.0 g of sample was weighed using an analytical balance (AUX320, Shimadzu, Kyoto, Japan). A total of 2.0 g of salt (NaCl and Na2SO4 in a 1:1 w/w ratio) was added, followed by an aliquot of the atrazine-d5 solution (surrogate standard) to yield a final concentration of 250 μg kg–1. A mixture of acetonitrile (8.0 mL) and ultrapure water (2.0 mL) was then added. The system was vortexed (K40-10208, Kasvi, São Paulo, Brazil) for 3.0 min at 2500 rpm and centrifuged (MPW-351R, MPW Med. Instruments, Warsaw, Poland) for 10.0 min at 15 °C and 10,000 rpm. After phase separation, approximately 6.0 mL of the supernatant was transferred to a Falcon tube containing 150.0 mg of PSA sorbent. The mixture was vortexed again for 3.0 min at 2500 rpm and centrifuged for an additional 10.0 min under the same conditions. The tubes were then placed in the freezer for 24 h. Once the aqueous phase had frozen, the organic extract containing the analytes was collected, filtered through a Cytiva Whatman MiniUniprep G2 vial with a 0.20 μm membrane filter (Marlborough, Massachusetts, USA), and injected into the GC–MS system (Figure 6).
 ACS Omega 2025, 10, 51, 63464–63473: Figure 6. Schematic representation of the extraction procedure using the QuEChERS–LTP procedure for pesticide extraction in cocoa powder samples.
ACS Omega 2025, 10, 51, 63464–63473: Figure 6. Schematic representation of the extraction procedure using the QuEChERS–LTP procedure for pesticide extraction in cocoa powder samples.
Results and Discussion
Evaluation of the Effect of Low and Ultralow Temperatures on the Analytical Response
Studies reporting the use of LTP generally employ a conventional freezer, which typically reaches −18 ± 2 °C as the minimum temperature. In this equipment, effective phase separation can require 8 to 24 h. (35−37) In contrast, ultralow temperature freezers (ultrafreezers) can reach −80 °C, leading to a substantial reduction in partitioning time. In this study, tests performed with the conventional freezer achieved satisfactory partitioning after 24 h, whereas in the ultrafreezer, complete partitioning was observed in only 20 min.
Considering the analytical response in terms of peak area (Figure 3), higher signals were obtained when LTP was performed in a conventional freezer rather than an ultrafreezer. This behavior can be attributed to the partitioning mechanism. (32) Partitioning depends on cooling and the subsequent freezing of the aqueous phase. In the conventional freezer, the slower freezing rate provides sufficient time for the system to approach thermodynamic equilibrium, favoring analyte diffusion between phases. Under these conditions, analyte molecules have more time to migrate and distribute optimally before complete solidification. In contrast, the rapid freezing in the ultrafreezer may trap analytes in a nonideal distribution, reducing the efficiency of the partitioning process (32) (Figure 3). According to these observations, the conventional freezer was selected for subsequent experiments.
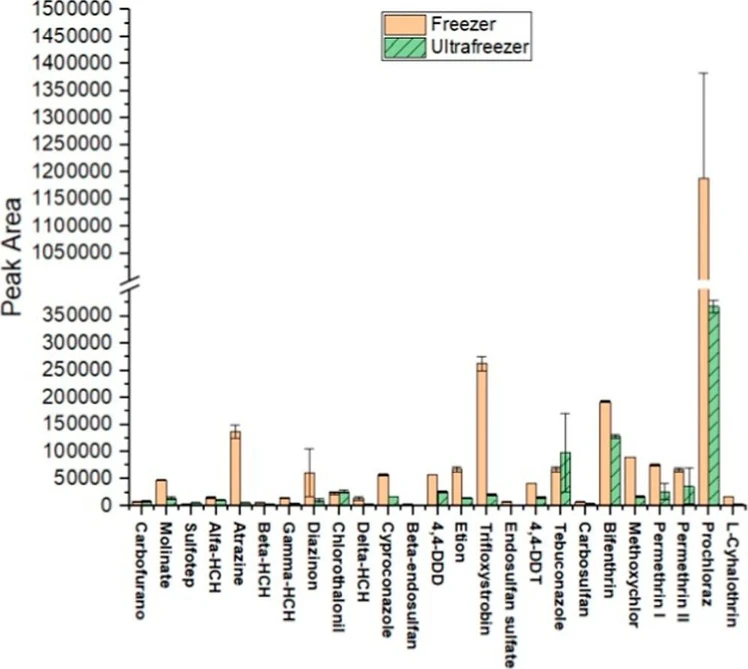 ACS Omega 2025, 10, 51, 63464–63473: Figure 3. Comparison of chromatographic peak areas of pesticides after partitioning in the conventional freezer versus the ultrafreezer.
ACS Omega 2025, 10, 51, 63464–63473: Figure 3. Comparison of chromatographic peak areas of pesticides after partitioning in the conventional freezer versus the ultrafreezer.
Application to Real Samples
Fifteen samples were analyzed in triplicate, comprising two from the State of Pará and 12 from the State of Bahia, located in the North and Northeast regions of Brazil, respectively. Among the 15 pesticides investigated, only permethrin isomers were quantified in four samples (Table 3). The herbicide molinate was also detected in three samples; however, its concentration was below LOQ. Permethrin isomers concentrations ranged from 17.1 ± 0.3 to 49.6 ± 1.8 μg kg–1. These values are below the MRL established by European Union regulation, which is 100 μg kg–1. (42) Although permethrin is not permitted in the European Union, (42) it remains authorized for use as insecticide in Brazil, particularly in crops such as cotton, rice, coffee, citrus, cabbage, beans, tobacco, maize, soybean, tomato, wheat, and grape. (13) However, no MRL values have been established for cocoa beans under Brazilian legislation. (13) Table 3 shows the concentration of detected compounds in the cocoa samples.
 ACS Omega 2025, 10, 51, 63464–63473: Table 3. Mean Concentrations (μg kg–1 ± Standard Deviation) of Target Pesticides Detected in This Study
ACS Omega 2025, 10, 51, 63464–63473: Table 3. Mean Concentrations (μg kg–1 ± Standard Deviation) of Target Pesticides Detected in This Study
To assess method performance for pesticides not detected in the cocoa samples, one of the analyzed samples was fortified with a mixed standard solution at concentrations of 50, 250, and 500 μg kg–1. The fortified analytes were subsequently detected in the chromatograms presented in Figures S2–S4, indicating good response of the proposed method.
Conclusions
The QuEChERS–LTP procedure was successfully developed for the determination of 16 multiclass pesticide residues in cocoa beans. For the first time, ultralow temperature (−80 °C) was applied to accelerate the low-temperature partitioning process during pesticide extraction from cocoa beans. Nevertheless, conventional freezing at −12 °C proved to be more effective for the extraction of the target analytes.
Evaluation of the matrix effect demonstrated that matrix-matched analytical curves, constructed in an analyte-free cocoa bean extract, were more effective in compensating for matrix interferences than the use of analyte protectants.
The pyrethroid permethrin was quantified in four cocoa samples. However, the concentrations did not exceed the MRL established by European Union legislation.
Overall, the QuEChERS–LTP procedure proved to be a promising approach for pesticide residue determination, as it requires smaller volumes of solvents and reagents, involves fewer cleanup and extraction steps, and shows strong potential for application to other complex matrices.