Synthesis and Identification of 3-Oxazolines in Cocoa
J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Graphical abstract
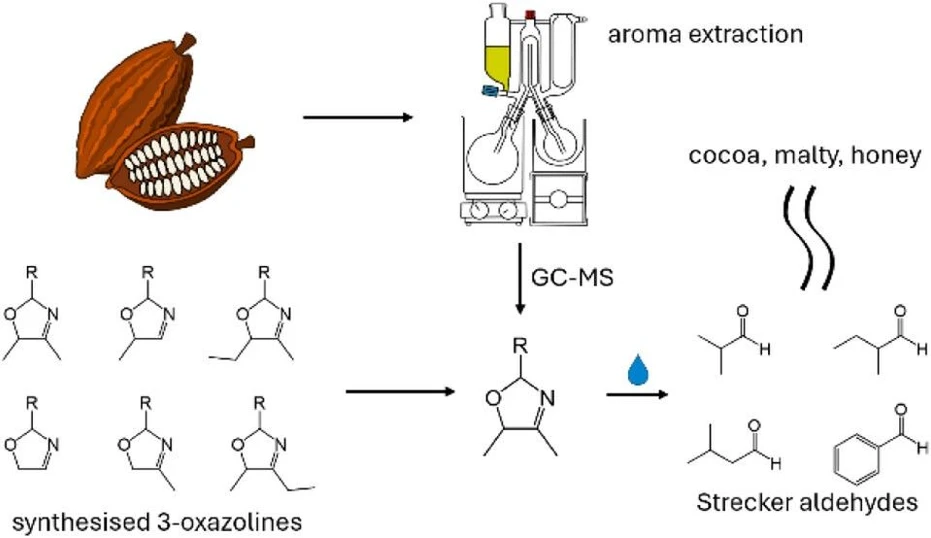
Adding water to chocolate strongly increases Strecker aldehydes, key cocoa aroma compounds. While 2-isobutyl-5-methyl-3-oxazoline was previously detected at low levels, other 3-oxazolines may serve as relevant precursors.
In this study, novel 3-oxazolines were synthesized and characterized using GC–MS and NMR. Four of these compounds were identified for the first time in aroma extracts of cacao nibs, cocoa liquor, and chocolate. These findings highlight 3-oxazolines as new contributors to cocoa aroma, offering potential strategies to enhance chocolate and other roasted food flavors.
The original article
Synthesis and Identification of 3-Oxazolines in Cocoa
Heather G. Spooner, Dimitris P. Balagiannis, Andreas Czepa, Barbara Suess, Martine Trotin, Paul O’Nion, and Jane K. Parker*
J. Agric. Food Chem. 2025, 73, 24, 15259–15269
https://doi.org/10.1021/acs.jafc.5c00898
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
3-Oxazolines have been proposed as precursors, present in cocoa, which hydrolyze to form Strecker aldehydes. Granvogl et al. (1) previously synthesized a range of 2-substituted-5-methyl-3-oxazolines and indeed showed their hydrolysis to Strecker aldehydes. However, upon searching for 2-isobutyl-5-methyl-3-oxazoline in dark chocolate, only a very low concentration was found.
Strecker aldehydes are key aroma compounds in roasted food products, such as chocolate, coffee, malt, and bread. (2,3) They are formed during the Maillard reaction by a variety of routes but most commonly via the Strecker degradation, which is the reaction of amino acids with α-dicarbonyl compounds. (4,5) Methionine, valine, leucine, isoleucine, phenylalanine, and alanine are six amino acids known to give rise to odor-active aldehydes. The α-dicarbonyl compounds, including methylglyoxal, 2,3-butanedione, or glucosone, arise by carbohydrate degradation, part of the Maillard reaction, or fermentation. (6) In addition, Amadori rearrangement products are formed in the early stages of the Maillard reaction by the condensation between an amino acid and a reducing sugar, and can act as key intermediates in the formation of α-dicarbonyls, as well as being direct precursors of Strecker aldehydes themselves. (7−10) There have also been further alternative routes to the classical Strecker degradation pathway identified, such as those that form “Strecker acids” and “Strecker amines”. (4,11) Thus, the 3-oxazolines are formed as part of the Maillard reaction, which is a highly complex web of reactions that gives rise to the compounds that color and flavor our food, of which Strecker degradation is just a part.
Several studies have reported that the addition of water to many dry food products, such as chocolate, cornflakes and malt, results in the release of a large amount of Strecker aldehydes. (2,12,13) Steam distillation of chocolate was found to increase the concentration of Strecker aldehydes, phenylacetaldehyde and 3-methylbutanal, by factors of ∼120 and ∼13, respectively. (14) Although Strecker aldehydes are generally thought to be thermally formed, Ullrich et al. found that chocolate made from unroasted cocoa beans also showed a high release of Strecker aldehydes upon water treatment, postulating that an alternative water-induced reaction pathway might exist to produce these compounds from “odorless precursors already present in the unroasted cocoa beans”. (15)
The first occurrence of 3-oxazolines in the literature was reported by Rizzi who identified 2-isopropyl-4,5-dimethyl-3-oxazoline during the Strecker degradation of valine with 2,3-butanedione under nonaqueous conditions. (16) More than 50 years later, Granvogl et al. investigated the role of 2-substituted-5-methyl-3-oxazolines as precursors of Strecker aldehydes, postulating their formation during Strecker degradation in the absence of water and remaining stable until the addition of water stimulates their hydrolysis, releasing the Strecker aldehyde. (1)
We propose that other 3-oxazolines may also be present in cocoa that are responsible for the release of Strecker aldehydes. The aim of this study was to synthesize a range of novel 3-oxazolines that we predict may be formed from known/relevant precursors and Maillard intermediates (Table 1), and search for their presence in cocoa.
Materials and Methods
Extraction and Identification of 3-Oxazolines in Cacao Nibs, Cocoa Liquor, and Chocolate
Identification by GC–MS
All samples of synthesized 3-oxazolines and SAFE extracts were analyzed by direct liquid injection (1 μL) by GC–MS. Due to the desire to run samples on columns of different polarities and to obtain accurate masses, many different GC–MS systems were used. For the nonpolar column, samples were manually injected in split mode 2:1, injector temperature 250 °C, flow 2 mL/min onto an HP-5 UI column (30 m × 0.25 mm × 1 μm; Agilent) with an Agilent 7890B/7250 GC-QToF system (Agilent, Santa Clara, CA). The oven temperature was held at 40 °C for 2 min, increased to 320 °C at a ramp of 4 °C/min, and held for 5 min. The carrier gas was helium at a flow rate of 1 mL/min. The mass spectrometer was used in both electron ionization (EI) mode and chemical ionization (CI) mode. In EI mode, the source temperature was 230 °C, ionization voltage 70 eV and scan range m/z 40 to m/z 210. In CI mode, the source temperature was 300 °C, ionization voltage 175 eV and scan range m/z 80 to m/z 220. The data were processed by using Agilent Masshunter software.
For the polar column, samples were manually injected (1 μL) in the splitless mode, with the injector at 250 °C, onto a ZB-Wax column (30 m × 0.25 mm × 1 μm; Phenomenex) with an Agilent 7890A/5975C GC–MS system as well as a DB-Wax UI column (30 m × 0.25 mm × 0.25 μm; Agilent) with an Agilent 6890/5975 GC–MS system. The oven temperature was increased from 40 to 250 °C at a ramp of 4 °C/min, and held for 10 min. The carrier gas was helium at a flow of 1 mL/min. Both mass spectrometers were operated in EI mode with a source temperature of 230 °C and ionization voltage of 70 eV. The scan range was from m/z 29 to m/z 400 (7890A/5975C system) or m/z 20 to m/z 400 (6890/5975 system). Selected ion monitoring (SIM) was applied for m/z 70, 84, 98, and 112 with a dwell time of 50 ms each. The data were processed by using Agilent MSD ChemStation.
In order to separate enantiomers, the synthesized 4,5-dimethyl-3-oxazolines (1–4) were run on a chiral column. Samples were injected in the splitless mode, with an injector temperature of 250 °C, onto a CP-Chirasil-Dex CB column (25 m × 0.25 mm × 0.25 μm; Agilent) with an Agilent 6890/5975 GC–MS system. The oven temperature was increased from 40 to 200 °C at a ramp of 4 °C/min and held for 20 min. The carrier gas was helium at a flow of 1 mL/min. The mass spectrometer was operated in EI mode with a source temperature of 230 °C, ionization voltage of 70 eV, and scan range of m/z 10 to m/z 400.
A series of n-alkanes (C5–C30) was also run under the same conditions in order to calculate the linear retention index (LRI) of each compound on each column.
Two-Dimensional Gas Chromatography–Mass Spectrometry (2D-GC–MS)
To obtain better separation of the compounds, a GC/GC–MS system was employed. The system consisted of a nonpolar HP-5MS 5% diphenyl column (30 m × 0.25 mm × 0.25 μm; Agilent) and a polar VF17 ms 17% diphenyl column (2 m × 0.1 mm × 0.2 μm; Agilent) with an Agilent 8890/7250 GC-QToF system and ZX2 thermal modulator (Zoex, Houston, TX). The oven temperature was increased from 50 to 300 °C at a ramp of 5 °C/min and held for 10 min. Samples were injected by an automatic liquid sampler in the split mode 5:1, injector temperature of 250 °C, helium flow of 4 mL/min onto the nonpolar column, and transferred to the polar column via a modulator column of deactivated silica (1 m × 0.1 mm; Zoex), formed into a double loop, which was periodically chilled at −90 °C for 5 s and heated at 450 °C for 300 ms. The mass spectrometer was used in EI mode with a source temperature of 200 °C, a scan range of m/z 33 to m/z 600, and an acquisition rate of 50 spectra/s. The data were processed by using a GC Image 2024 GCxGC.
Gas Chromatography–Olfactometry (GC-O)
Synthesized compounds 1–4 were analyzed for odor characteristics by GC-O. Samples were manually injected (1 μL) in splitless mode, with the injector at 250 °C, onto an HP-5MS UI column (30 m × 0.25 mm × 0.25 μm; Agilent) with an Agilent 7890B GC equipped with a flame ionization detector (Hewlett-Packard, Waldbronn, BaWü, Germany) and an ODO II odor port (SGE, Ringwood, Victoria, Australia). The oven temperature was increased from 40 to 200 °C at a ramp of 4 °C/min, then increased to 300 °C at a ramp of 8 °C/min, and held for 8 min. The carrier gas was helium at a flow of 2 mL/min. The column effluent was split equally between the FID and odor port, where the odors of the eluting compounds were evaluated on the basis of matching LRI by 5 assessors.
A series of n-alkanes (C5–C30) was also run under the same conditions in order to calculate the linear retention index (LRI) of each compound.
NMR Spectroscopy
1H, 13C, COSY, HSQC, and HMBC NMR spectra were recorded by using a Bruker Nanobay 400 MHz spectrometer (Bruker, Rheinstetten, Germany). For preparation, samples (1 mL) were evaporated to dryness under a gentle stream of nitrogen, and CDCl3 (0.7 mL) was added. The data were processed using Bruker TopSpin 4.4.0, and chemical shifts were determined using the proton signal (7.26 ppm; 1H NMR) or carbon signal (77.0 ppm; 13C NMR) of CDCl3.
Results and Discussion
Identification of 4,5-Dimethyl-3-oxazolines (1–4) in Cocoa Products
The presence of these 4,5-dimethyl-3-oxazolines was then investigated in cocoa and chocolate. Ground, roasted cacao nibs were subjected to SAFE distillation, and the concentrated aroma extract was analyzed by GC–MS. Using the synthesized 4,5-dimethyl-3-oxazolines as standards, 2-isopropyl-, 2-isobutyl-, 2-sec-butyl-, and 2-benzyl-4,5-dimethyl-3-oxazoline (1–4) were identified in cocoa for the first time. This identification was supported by the matching of LRI values on both the nonpolar and polar columns, identical exact masses by CI ± 10 ppm, and the same EI mass spectrum (Figure 4). In the case of 2-benzyl-4,5-dimethyl-3-oxazoline (4), only a very low signal was found; therefore, selected key m/z were used for mass spectrum confirmation. However, in the case of 1–3, the signals detected were larger, and more accurate mass spectra were obtained. Due to complexity of the 1D-GC-MS chromatogram, the SAFE extract was also analyzed by 2D-GC–MS for better separation of the compounds. Again, comparison with the synthesized standards allowed the identification of the same 4,5-dimethyl-3-oxazolines (1–4) on the basis of matching retention times and mass spectra (Figure 5).
 J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 4. GC–MS data supporting the identification of synthesized 3-oxazolines in cocoa. Mass spectrum provided is from the larger of the two peaks, both of which have similar fragmentation patterns.
J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 4. GC–MS data supporting the identification of synthesized 3-oxazolines in cocoa. Mass spectrum provided is from the larger of the two peaks, both of which have similar fragmentation patterns.
 J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 5. 2D-GC–MS analysis of roasted cacao nibs, extracted by SAFE. The synthesized 4,5-dimethyl-3-oxazoline standards 1–4 were each run separately and used to create a template (indicated by the green, blue, red, and orange circles, respectively) to show the expected retention times of 4,5-dimethyl-3-oxazolines. This image was created by extracting ion 98 from the chromatogram of the SAFE extract and overlaying with the template. Compounds in cocoa, shown here as blobs, were found close to or within the template regions, and possessed the same mass spectrum as the synthesized standards, indicating the presence of these 4,5-dimethyl-3-oxazolines in cocoa for the first time.
J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 5. 2D-GC–MS analysis of roasted cacao nibs, extracted by SAFE. The synthesized 4,5-dimethyl-3-oxazoline standards 1–4 were each run separately and used to create a template (indicated by the green, blue, red, and orange circles, respectively) to show the expected retention times of 4,5-dimethyl-3-oxazolines. This image was created by extracting ion 98 from the chromatogram of the SAFE extract and overlaying with the template. Compounds in cocoa, shown here as blobs, were found close to or within the template regions, and possessed the same mass spectrum as the synthesized standards, indicating the presence of these 4,5-dimethyl-3-oxazolines in cocoa for the first time.
For additional confirmation of the presence of these compounds in cocoa, the synthesized and purified 4,5-dimethyl-3-oxazolines were spiked into the cacao nib SAFE extract and analyzed by 1D-GC–MS. The peaks attributed to 3-oxazolines were observed to increase, confirming that 1–3 were indeed present. This also allowed the peaks for 2-isobutyl- and 2-sec-butyl-4,5-dimethyl-3-oxazoline to be separated, which are previously undifferentiable due to their similar LRI values (Figure S3).
To search for 3-oxazolines in other cocoa products, aroma extracts of cocoa liquor, dark chocolate, and milk chocolate were obtained by SAFE distillation. Compounds 2 and 3 were consistently identified in all of the samples by 1D-GC–MS; however, 2D-GC–MS analysis enabled confident identification of 1 (in milk chocolate) and 4 (in cacao nibs, cocoa liquor, and milk chocolate) (Figure S4). For dark chocolate, which was not analyzed by 2D-GC–MS, 1 and 4 were tentatively identified by 1D-GC–MS (Table 4). Further work may carry out quantification of these compounds to investigate the effects of cocoa processing.
Synthesis of Other 3-Oxazolines
In this study, we also speculated about the possibility of 3-oxazolines derived from other α-dicarbonyl compounds being present in cocoa. We synthesized 4,5-unsubstituted-3-oxazolines, 5-methyl-3-oxazolines, 4-methyl-3-oxazolines, and 4/5-ethyl-5/4-methyl-3-oxazolines, theoretically generated from glyoxal, methylglyoxal, and 2,3-pentanedione, respectively (Table 1). Methylglyoxal and 2,3-pentanedione are both asymmetric compounds, so they were proposed to react with amino acids in two orientations to form 3-oxazoline regioisomers, with methylglyoxal forming 5-methyl- and 4-methyl-3-oxazolines (Figure 6), and 2,3-pentanedione forming 5-ethyl-4-methyl- and 4-ethyl-5-methyl-3-oxazolines.
 J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 6. Methylglyoxal is an asymmetrical α-dicarbonyl compound and was proposed to react with amino acids, such as valine, in two conformations to generate 3-oxazoline regioisomers, 2-isopropyl-5-methyl-3-oxazoline and 2-isopropyl-4-methyl-3-oxazoline, in the case of valine (9 and 13, respectively). In the same way, 2,3-pentanedione can also react to form the regioisomers, 5-ethyl-4-methyl- and 4-ethyl-5-methyl-3-oxazolines.
J. Agric. Food Chem. 2025, 73, 24, 15259–15269: Figure 6. Methylglyoxal is an asymmetrical α-dicarbonyl compound and was proposed to react with amino acids, such as valine, in two conformations to generate 3-oxazoline regioisomers, 2-isopropyl-5-methyl-3-oxazoline and 2-isopropyl-4-methyl-3-oxazoline, in the case of valine (9 and 13, respectively). In the same way, 2,3-pentanedione can also react to form the regioisomers, 5-ethyl-4-methyl- and 4-ethyl-5-methyl-3-oxazolines.
Due to the limitations in the availability of reagents, alternative synthetic methods were used (Figure 1), and the purity of the compounds was significantly lower than that of the compounds 1–4, making these compounds very difficult to isolate. The regioisomers arising from methylglyoxal (9–16) were able to be individually synthesized and identified due to the amino alcohol asymmetry and therefore were given different compound numbers. However, the regioisomers of 17–20 were both synthesized from 2,3-pentanedione where there was no regioselective control; therefore, these compounds were labeled with the same compound number but as “a” or “b”. More isomers of 19a/b were observed due to the extra chiral center of the sec-butyl group, possibly leading to the stereoisomers being more spatially different and therefore able to be distinguished by the achiral GC–MS column.
Granvogl et al. previously demonstrated the adaptation of their 3-oxazoline synthetic method by varying the amino alcohol reagent, and this was structurally confirmed by NMR. (1) Our synthesis was simply an extension of this; therefore, 5–16 were tentatively identified on the basis of their mass spectra matching the expected fragmentation pattern (Figure S5), their expected exact mass values being observed by CI mass spectrometry (Table 2), and the fact that analogues prepared by the same synthetic methods had previously been fully characterized by NMR. Compounds 17a/b–20a/b were synthesized by modifying Rizzi’s method, substituting the α-dicarbonyl compound 2,3-butanedione for 2,3-pentanedione. The purification and NMR analysis of regioisomers 17a and 17b was attempted, although the isomers could not be separated by column chromatography. The NMR spectrum showed the presence of 2-isopropyl-5-ethyl-4-methyl-3-oxazoline as the major isomer, with the position of the ethyl group on C5 confirmed by the COSY and HMBC experiments. The integrals of the four multiplet signals of H2 and H5, indicative of 3-oxazolines, suggested the presence of the minor regioisomer, 2-isopropyl-4-ethyl-5-methyl-3-oxazoline; however, this could not be confirmed due to the presence of impurities (Figure S6). After ∼5 days of storage of the samples, 2-isopropyl-5-ethyl-4-methyloxazole was observed by GC–MS (confirmed with the NIST Chemistry WebBook (19)), likely caused by oxidation of the synthesized oxazoline, further supporting evidence that the 5-ethyl-4-methyl-3-oxazoline was the major isomer. Use of the same synthetic method, varying just the amino acid, to generate 18a/b–20a/b, as well as the expected mass fragmentation and exact molecular mass being observed, provided enough evidence for us to conclude (albeit tentatively) that 5/4-ethyl-4/5-methyl-3-oxazolines had also been synthesized.