Optimization of 1,4-Dioxane and Ethanol Detection Using USEPA Method 8260

- Photo: EST Analytical: Optimization of 1,4-Dioxane and Ethanol Detection Using USEPA Method 8260
- Video: EST Analytical: EST Evolution
During the manufacture of pharmaceuticals, 1, 4-Dioxane is used as a cleansing agent. It is also a byproduct of plastic manufacturing. The most likely exposure to 1, 4-Dioxane is at an industrial site. However, if any 1, 4-Dioxane is released into the environment during manufacturing there is a potential for it to migrate into ground water. The fact that 1, 4-Dioxane is so miscible in water makes degradation of the chemical challenging. Ethanol, on the other hand, has been a popular gasoline additive. Since it burns more quickly and completely than gasoline, emissions from car exhaust are decreased. The downside of this is underground storage tank leakage and fuel spills cause ground water and drinking water to be contaminated with both the fuel and the ethanol additive. Since ethanol is also very miscible in water, the detection of ethanol contamination in water can be difficult.
There have been several innovations that help overcome the obstacles of detecting 1, 4-Dioxane and Ethanol. The first one is the SIM mode of the mass spectrometer. This mode allows better detection of hard to extract compounds. Furthermore, mass spectrometers are now equipped to run SIM/Scan, this not only helps detect more difficult compounds, but also enables the detection of an extensive list of USEPA Method 8260 compounds without having to run the samples twice. Advancements in purge and trap sampling have also facilitated better detection of these compounds. Most effective in this has been the ability to heat the samples. However, there are drawbacks to better detection. The most problematic of these is the tendency of 1, 4-Dioxane and Ethanol to “stick” to the glass ware. This susceptibility has caused many headaches in environmental labs.
This application note will investigate seven variations of purge and trap sampling. The data will then be evaluated in order to recommend the optimum purge and trap sampling parameters for your lab.
Experimental:
The sampling system used for this study was the EST Analytical Evolution concentrator affixed with a Vocarb 3000 trap. The Centurion WS autosampler equipped with the syringe option was employed as the autosampler. The separation and analysis were performed by an Agilent 7890A Gas Chromatograph (GC) and 5975C inert XL Mass Spectrometer (MS). The GC was configured with a Restek Rxi-624 Sil MS 30m x 0.25mm x 1.4µm column. The purge and trap and GC/MS parameters used for this study are listed below.
Purge and Trap Parameters
- Concentrator: EST Encon Evolution
- Trap type: Vocarb 3000
- Valve oven temperature: 150 °C
- Transfer line temperature: 150 °C
- Trap temperature: 35 °C
- Moisture Reduction Trap (MoRT) temperature: 39 °C
- Purge time: 11 min
- Purge flow: 40 mL/min
- Dry purge: Ambient temperature, 40 mL/min, 1.0 min
- Desorb pressure: 5 psi (with control ON)
- Desorb time: 0.5 min
- Desorb preheat delay: 10 sec
- Desorb temperature: 250 °C
- MoRT bake temperature: 210 °C
- Bake temperature: 260 °C
- Sparge vessel bake temperature: 40 °C for 3 min, ramp to 100–110 °C, hold
- Bake time: 6 min
- Bake flow: 85 mL/min
Auto-Sampler (EST Centurion WS):
- Sample type: Water
- Sample volume: 5 or 10 mL
- Internal standard volume: 5 µL
GC/MS Parameters
- System: Agilent 7890A GC / 5975C inert XL MS
- Inlet: Split mode, 220 °C, 40:1 split ratio, 12.153 psi head pressure
- Column: Restek Rxi-624Sil MS, 30 m × 0.25 mm × 1.4 µm
- Oven program: 45 °C (1 min) → ramp 15 °C/min → 220 °C, hold 1.33 min (total run ~14 min)
- Column flow rate: 1 mL/min helium
- Total flow: 44 mL/min
- Source temperature: 230 °C
- Quadrupole temperature: 150 °C
- Transfer line temperature: 180 °C
- Scan range: m/z 35–300, 5.2 scans/sec
- SIM ions:
- 45, 46 (0.7–3.49 min)
- 58, 88 (3.5–14 min)
- Solvent delay: 0.7 min
For each parameter iteration, see the text below, a calibration curve was established with a linear range of 0.5 to 200ppb using standards from Restek. After each of the prescribed curves was determined; Method Detection Limits (MDLs), precision and accuracy and carryover studies were performed. A series of seven low level standards were run in order to establish MDLs per 40CFR Part 136, Appendix B. Next, seven replicate samples of the 50ppb standard were run in order to establish the precision and the accuracy of the experimental parameters. Finally, a series of four 50ppb standards were run with each standard followed by three blanks. The amount of carryover was then analyzed in the first blank. The data from each parameter variation was compiled and compared in order to determine the optimum conditions for this analysis. An average of the results for each analysis condition is listed in Table 1.
Purge and Trap Sampling Test Parameters
- Baseline: 5 mL traditional sparger, room temp purge, 110 °C sparge bake
- Iteration A: 5 mL traditional sparger, 40 °C purge, 110 °C sparge bake
- Iteration B: 5 mL traditional sparger, 60 °C purge, 110 °C sparge bake
- Iteration C: 5 mL traditional sparger, 40 °C purge, no sparge bake
- Iteration D: 40 mL vial, 10 mL purge volume, 40 °C purge, no sparge bake
- Iteration E: 5 mL fritless bulbless sparger, 40 °C purge, 110 °C sparge bake
- Iteration F: 5 mL fritless bulbless sparger, 40 °C purge, no sparge bake
(Iteration E used the patented Water/Soil prep mode)
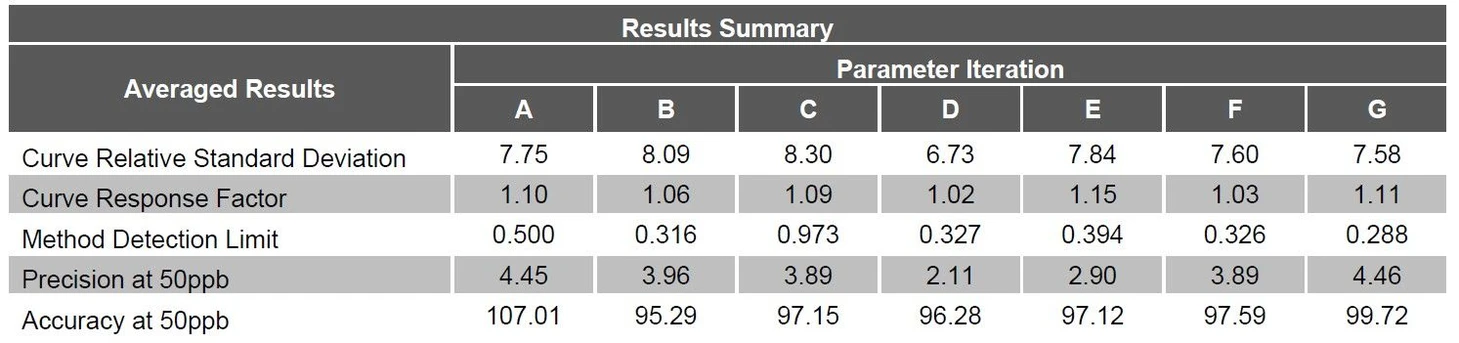 EST Analytical: Table 1 Averaged Results Summary
EST Analytical: Table 1 Averaged Results Summary
The primary compounds of interest for the study were Ethanol and 1, 4-Dioxane. For this reason, the data for these compounds was compiled separately in order to better distinguish the benefits or detriment of each purge and trap parameter set. The experimental results of the precision and percent recovery studies are displayed in Tables 2 and 3.
 EST Analytical: Table 2 Ethanol and 1, 4-Dioxane Precision at 50ppb
EST Analytical: Table 2 Ethanol and 1, 4-Dioxane Precision at 50ppb
 EST Analytical: Table 3 Ethanol and 1, 4-Dioxane Percent Recovery at 50ppb
EST Analytical: Table 3 Ethanol and 1, 4-Dioxane Percent Recovery at 50ppb
For the carryover studies, Ethanol and 1, 4-Dioxane were examined along with the carryover of 1, 2, 4- Trichlorobenzene, Naphthalene and 1, 2, 3-Trichlorobenzene. The reason the heavier compounds were included in this study was a concern that changing the sampling parameters may help the Ethanol and 1, 4-Dioxane carryover, but harm the carryover of the heavier compounds of interest. Table 4 shows a listing of the carryover results, in parts per billion, while Figure 1 displays the percent carryover of the different purge and trap experimental parameters for just the Ethanol and 1, 4-Dioxane.
 EST Analytical: Table 4 Carryover in First Blank after a 50ppb Standard
EST Analytical: Table 4 Carryover in First Blank after a 50ppb Standard
 EST Analytical: Figure 1 Ethanol and 1, 4-Dioxane Percent Carryover after a 50ppb Standard Graphic
EST Analytical: Figure 1 Ethanol and 1, 4-Dioxane Percent Carryover after a 50ppb Standard Graphic
After all the studies were completed, two purge and trap parameter variations showed the best precision, percent recovery, and carryover while meeting the USEPA Method 8260 linearity and response factor requirements. An abbreviated listing of the results for these two iterations are shown in Table 5.
 EST Analytical: Table 5 Results of the Water Extraction and the Fritless - Bulbless Sparge Vessel With Sparge Bake Studies
EST Analytical: Table 5 Results of the Water Extraction and the Fritless - Bulbless Sparge Vessel With Sparge Bake Studies
Conclusions:
All seven purge and trap parameter iterations passed the USEPA Method 8260 requirements for linearity and method detection limits. The problem with some of the experimental parameters was found in the carryover and precision and accuracy studies. The carryover using the traditional sparge vessel showed a large amount of carryover for the Ethanol and 1, 4-Dioxane. Since the carryover was so high, the precision and accuracy data suffered. The Fritless/Bulbless sparge vessel, on the other hand, displayed much lower carryover for the Ethanol and 1, 4-Dioxane especially when the patented sparge bake was not used. However, the 1, 2, 4-Trichlorobenzene, Naphthalene and 1, 2, 3-Trichlorobenzene carryover after the 50ppb standard was above the lower limit of the curve.
Thus, the sparge bake would be recommended in order to limit the carryover of the heavier compounds. The optimum purge and trap parameters proved to be the patented water extraction technique. This technique provided linearity and method detection limits that met the USEPA Method 8260 requirements, while providing excellent precision and accuracy data. During water extraction, the sample is transferred to an empty 40ml vial and then purged in the soil station of the Centurion. The “fresh” vial provides a clean sparge vessel for every sample thus limiting carryover for both the Ethanol and 1, 4-Dioxane and for the heavy compounds. This lack of carryover aided in providing optimum precision and accuracy and carryover results and would be the recommended method for examining these troublesome compounds.
- Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); United States Environmental Protection Agency Method 8260C, Revision 3, August, 2006.
- Jurek, Anne, “A Single Calibration Method for Water AND Soil Samples Performing EPA Method 8260”, https://www.estanalytical.com/a-single-calibration-method-for-water-and-soil-samples/, EST Analytical, published 3, 2015, Web 4, 2016.