Quality control in GC–MS analysis of amino acids in human urine and plasma: Possible implications for targeted and untargeted metabolomics
Journal of Chromatography B, Volume 1262, 2025, 124661: Graphical abstract
The aim of the study is to evaluate a previously established quality control (QC) approach for targeted GC–MS analysis of amino acids in human plasma and apply it to human urine samples. Using isotope-labeled internal standards and precise derivatization techniques, the study investigates the accuracy, precision, and robustness of the method under closely matched conditions for both urine and plasma samples.
The results demonstrate that while untargeted GC–MS metabolomics may not yield reliable quantitative data for amino acids, targeted approaches using isotopologs are indispensable for accurate measurements. The findings support the broader implication that such QC-based targeted GC–MS methods are critical for reliable metabolomic analyses in various biological matrices.
The original article
Quality control in GC–MS analysis of amino acids in human urine and plasma: Possible implications for targeted and untargeted metabolomics
Alexander Bollenbach, Bibiana Beckmann, Dimitrios Tsikas
Journal of Chromatography B, Volume 1262, 2025, 124661
https://doi.org/10.1016/j.jchromb.2025.124661
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Mass spectrometry (MS)-based technologies including LC-MS, LC-MS/MS, GC–MS and GC–MS/MS are widely used in targeted and untargeted metabolomics studies for the analysis of various classes of metabolites in biological samples such as plasma, serum and urine (Table 1). Metabolomics studies are also widely performed on α-amino acids. Table 1 indicates that amino acids are an important class of organic analytes studied by the so-called OMICS techniques. Amino acids contain one or two carboxylic acids and at least one primary (NH2) or secondary (NH) amine group as exemplarily shown for L-arginine in Fig. 1. In urine, amino acids occur mostly in their free form. Amino acids are soluble in water and in water-miscible organic solvents such as methanol. In aqueous solutions, amino acids are charged at any pH value, i.e., they are zwitterionic, with their carboxylic group(s) being deprotonated and their amine group(s) being protonated (Fig. 1). GC–MS analysis of amino acids requires their conversion to charge-free lipophilic derivatives that are readily extractable into GC-compatible, water-immiscible organic, and are volatile and thermally stabile in solvents such as toluene [[1], [2], [3], [4]] (Fig. 1).
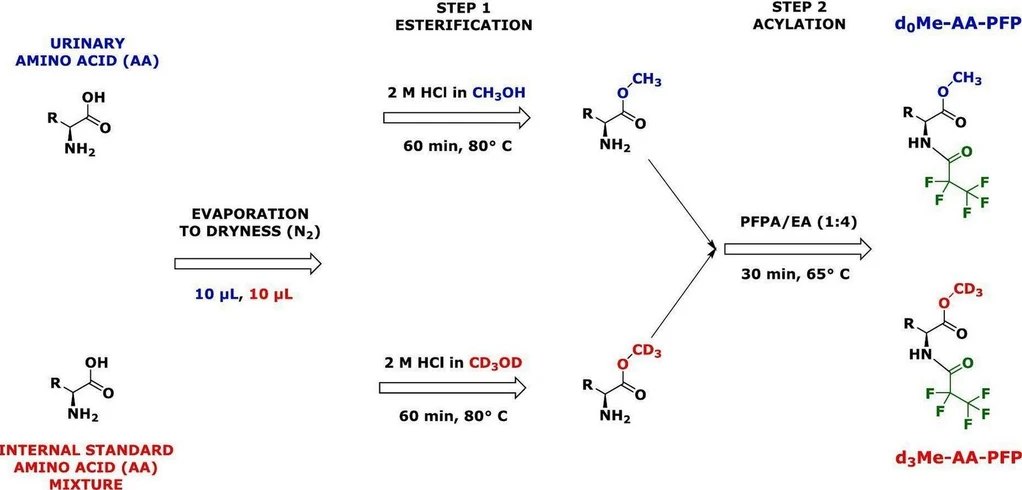
Amino acids (AA) can be specifically converted to their methyl esters (Me) by heating in 2 M HCl in methanol (CH3OH) or deuterated methanol (CD3OD) [5]. The reaction products are unlabeled methyl (Me) esters, i.e., d0Me-AA, and deuterated Me, i.e., d3Me-AA. Amine/imine groups of amino acids are subsequently acylated by using perfluorinated organic anhydrides such as pentafluoropropionic (PFP) anhydride (PFPA) (Fig. 2) [2,5]. The two-step derivatization procedure enables in-situ preparation of d3Me-AA for use as internal standards (IS) in quantitative (targeted) GC–MS analyses [6] (Fig. 2). These derivatization procedures and the long-term stability of the Me-PFP derivatives in toluene [7] allow for a high-throughput stable-isotope dilution GC–MS analysis of amino acids in biological samples (e.g., urine and plasma) in OMICS-format and at low costs. The utility of the GC–MS method for the high-throughput quantitative measurement of amino acids in human plasma samples in clinical and experimental studies has been reported by our group (e.g., [[5], [6], [7], [8]]).
Quality control (QC) systems are essential in analytical chemistry in general and in OMICS studies in particular [[9], [10], [11], [12], [13], [14], [15], [16], [17]]. Table 1 indicates that QC is often applied in metabolomics studies involving amino acid analysis. In previous work, we reported on the development and application of a QC system for the targeted metabolomics GC–MS analysis of amino acids as Me-PFP derivatives in human plasma [8]. In OMICS studies, urine is a considerably less often analyzed matrix compared to serum and plasma (Table 1). The aim of the present work was to adapt such a QC system for amino acids analysis in human urine. The main principle of the targeted GC–MS analysis of urinary amino acids for their high-throughput quantitative determination in small-volume human urine and plasma samples in clinical settings is depicted schematically in Fig. 2. It is based on the in-situ preparation of a mixture of desired d3Me-AA for use as IS [5], in parallel with the esterification of biological amino acids to their d0Me-AA derivatives and their subsequent conversion to their pentafluoropropionyl (PFP) derivatives, i.e., d3Me-AA-PFP and d0Me-AA-PFP, respectively.
Another aim of the work was also to investigate and compare GC (e.g., retention time) and MS (e.g., detector response) effects in urine and plasma samples from a clinical study including QC urine samples. On the basis of these results, possible implications for targeted and untargeted metabolomics of amino acids in human urine and plasma were discussed.
2. Experimental
2.5. GC–MS conditions and calculations
Analyses were performed on a GC–MS apparatus consisting of a single quadrupole mass spectrometer model ISQ, a Trace 1210 series gas chromatograph and an AS1310 autosampler from ThermoFisher (Dreieich, Germany). A fused-silica capillary column Optima 17 (15 m length, 0.25 mm I.D., 0.25 μm film thickness) from Macherey-Nagel (Düren, Germany) was used. Aliquots (1 μL) of toluene extracts were injected in the splitless mode. The injector temperature was kept at 280 °C. Helium was used as the carrier (1.0 mL/min). The oven temperature was held at 40 °C for 0.5 min and ramped to 210 °C at a rate of 15 °C/min and then to 320 °C at a rate of 35 °C/min. Interface and ion-source temperatures were set to 300 °C and 250 °C, respectively. Electron energy was 70 eV and electron current 50 μA. Methane (2.4 mL/min) was used as the reagent gas for negative-ion chemical ionization (NICI). Quantitation was performed in the selected-ion monitoring (SIM) mode (Table 2). By this GC–MS method, the sum of Cit and Orn (Orn/Cit), the sum of Asn and Asp (Asp/Asn), the sum of Glu and Gln (Glu/Gln), and the sum of Leu and Ile (Leu/Ile) is measured independent of the samples [6].
3. Results
3.1. Amino acids concentrations in quality control and study urine samples
The concentrations of the amino acids in their stock solutions used in the present work had been standardized as described recently for the targeted GC–MS analysis in human plasma samples [8]. Recall that by this GC–MS method, the sum of Cit and Orn (Orn/Cit), the sum of Asn and Asp (Asp/Asn), and the sum of Glu and Gln (Glu/Gln), due to conversion of Cit to Orn, of Asn to Asp, and of Gln to Glu, is measured [6]. The sum of Leu and Ile (Leu/Ile) is measured due to coelution of the isobaric Leu and Ile. The concentrations of the amino acids determined in the QC urine samples are reported in Table 3.
 Journal of Chromatography B, Volume 1262, 2025, 124661: Table 3. Mean concentrations (C, μM), recovery (R, %) and coefficient of variation (CV, %) of the measurement of the listed amino acids (AA) in the four QC urine samples. Recovery is not applicable to QC1.
Journal of Chromatography B, Volume 1262, 2025, 124661: Table 3. Mean concentrations (C, μM), recovery (R, %) and coefficient of variation (CV, %) of the measurement of the listed amino acids (AA) in the four QC urine samples. Recovery is not applicable to QC1.
4. Discussion
The occurrence of biological amino acids and their metabolites in wide concentration ranges may represent a considerable analytical challenge for their simultaneous quantitative measurement. Only MS-based analytical instrumental techniques such as GC–MS and LC-MS/MS utilize isotopically labelled amino acids (isotopologs) as internal standards for accurate quantitative analysis [24,25,26]. Nowadays, synthetic amino acids labelled with a sufficient number of stable isotopes (2H, 13C, 15N) are commercially available, albeit costly. The concentrations of the amino acids that were measured in human urine and plasma samples by our group (e.g., [[5], [6], [7], [8]]) are within ranges reported by other groups (e.g., [2,3,4,[27], [28], [29], [30], [31], [32]]) by using different analytical methods including GC–MS and LC-MS/MS. Our group has proposed a practical and cost-saving in-situ preparation method for trideutero-methyl esters (AA-d3Me) from commercially available unlabeled amino acids [5]. This method is useful for the preparation of a mixture of desired AA-d3Me that enables reliable quantitative GC–MS analysis of amino acids in biological samples (Fig. 2).
In GC–MS- and LC-MS-based untargeted metabolomics, analytical shortcomings may result from many sources, among others from instrumental drifts [33]. Two kinds of factors have been recognized so far and include 1) GC-related factors, notably shifts in retention time, and 2) MS-related factors, especially variation of detection response. These types of errors and the utility of so-called intrastudy quality control (QC) samples have been recently reviewed and discussed, and helpful recommendations have been proposed [33].