GC & GC/MS webinars Week 26/2022
LabRulez: GC & GC/MS webinars Week 26/2022
In the week from June 27, 2022, the following webinars await you in the field of GC and GC/MS.
👉 DETAILS AND REGISTRATION IN THE WEBINARS SECTION
👉 Search and filter in almost 2 000 GC, LC and MS webinars.
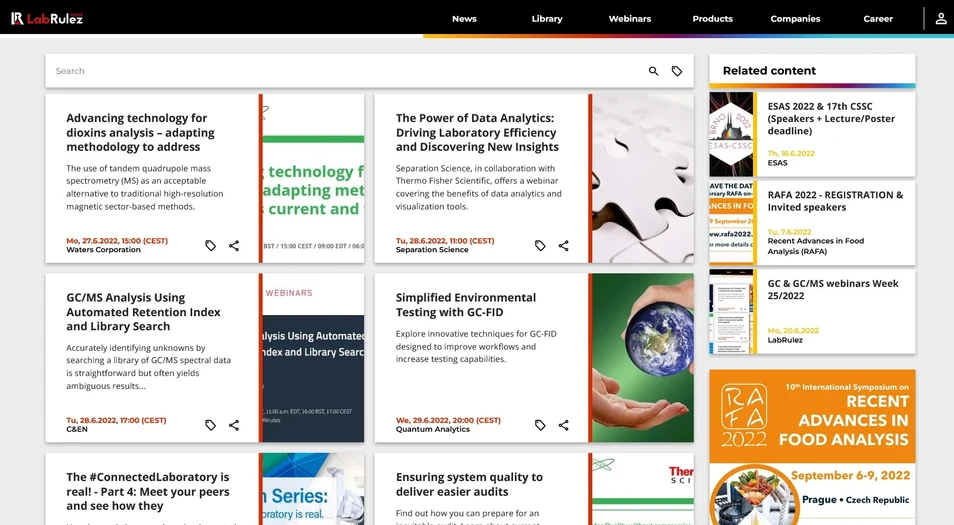
1. Waters Corporation: Advancing technology for dioxins analysis – adapting methodology to address current and future needs
- Mo, 27.6.2022, 15:00 (CEST)
The use of tandem quadrupole mass spectrometry (MS) as an acceptable alternative to traditional high-resolution magnetic sector-based methods.
2. Separation Science/Thermo Fisher Scientific: The Power of Data Analytics: Driving Laboratory Efficiency and Discovering New Insights
- Tu, 28.6.2022, 11:00 (CEST)
Separation Science, in collaboration with Thermo Fisher Scientific, offers a webinar covering the benefits of data analytics and visualization tools.
3. C&EN/Cerno Bioscience: GC/MS Analysis Using Automated Retention Index and Library Search
- Tu, 28.6.2022, 17:00 (CEST)
Accurately identifying unknowns by searching a library of GC/MS spectral data is straightforward but often yields ambiguous results...
4. Quantum Analytics/Activated Research Company: Simplified Environmental Testing with GC-FID
- We, 29.6.2022, 20:00 (CEST)
Explore innovative techniques for GC-FID designed to improve workflows and increase testing capabilities.
5. Waters Corporation: The #ConnectedLaboratory is real! - Part 4: Meet your peers and see how they implemented the #ConnectedLaboratory
- Th, 30.6.2022, 13:30 (CEST Prague)
Hear how existing users have implemented the #ConnectedLaboratory.
6. SelectScience/Thermo Fisher Scientific: Ensuring system quality to deliver easier audits
- Th, 30.6.2022, 16:00 (CEST)
Find out how you can prepare for an inevitable audit. Learn about current regulatory requirements and what this means for your lab. Discover expert hints and tips for an effective system review.