California Oxygenates and 8260
EST Analytical: California Oxygenates and 8260
For more than 30 years, oxygenated compounds have been added to gasoline. The use of oxygenates provides two primary benefits: a reduction in pollution caused by vehicle emissions, as oxygenated gasoline burns more efficiently, and an improvement in overall engine performance.
However, the prolonged use of oxygenated compounds has also led to environmental concerns. Increased contamination of groundwater has been observed, primarily due to leaks from underground storage tanks, allowing oxygenates to migrate into soil and water. In addition, because these compounds are highly soluble in water, analytical methods must be carefully optimized to achieve adequate detection limits.
Discussion
This application note investigates purge-and-trap experimental conditions to determine the most efficient and accurate approach for purging highly soluble fuel oxygenates. The study focuses on evaluating the effects of sample volume and purge temperature on analytical performance.
The parameters examined include purge volumes of 5 mL, 10 mL, and 25 mL, as well as purge temperatures at room temperature and 60 °C. Experimental results were compared in terms of calibration range, linearity, method detection limits (MDLs), and compound response.
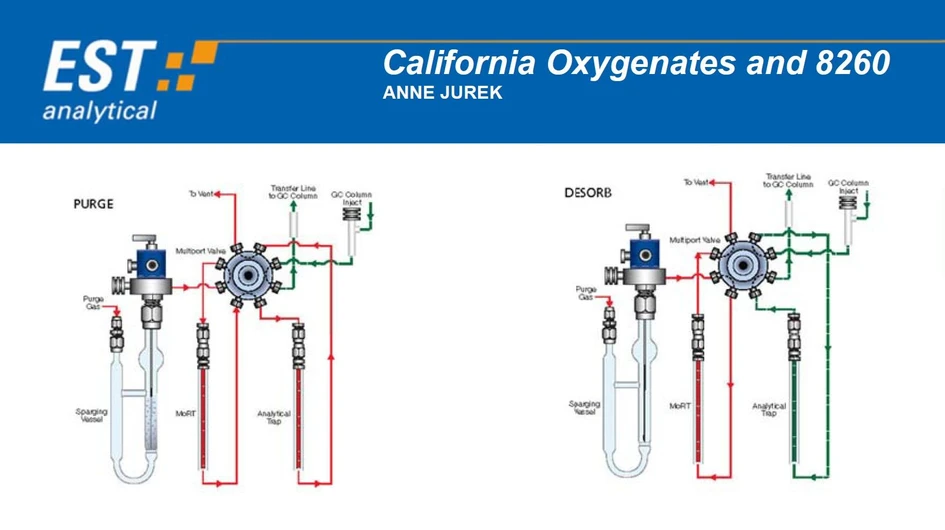
A key feature of this study is the use of the EST Encon Evolution purge-and-trap concentrator, which provides enhanced control over moisture introduction into the GC system. Unlike conventional concentrators, the Encon Evolution employs an 8-port valve design that transfers analytes directly from the analytical trap to the GC via the transfer line, while excluding the Moisture Reduction Trap (MoRT) from the desorption pathway. This design is particularly advantageous when operating at elevated purge temperatures, as was required in this study. Flow paths for purge and desorption are illustrated in Figures 1 and 2.
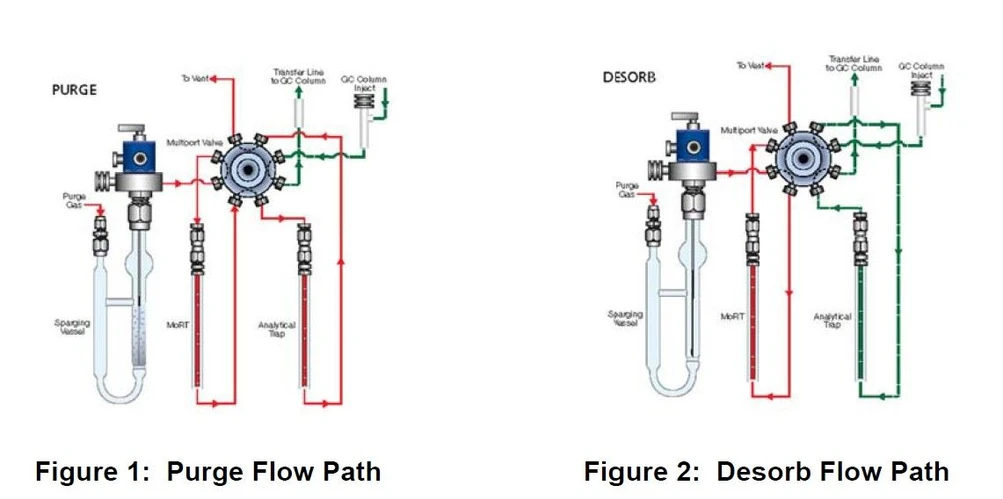 EST Analytical: Figure 1 & 2: Purge and Desrob Flow Path
EST Analytical: Figure 1 & 2: Purge and Desrob Flow Path
Experimental
Instrumentation
The sampling and concentration system consisted of an EST Analytical Centurion WS autosampler coupled to an EST Encon Evolution purge-and-trap concentrator. Experiments were conducted in water mode.
The concentrator was configured with a VOCARB 3000 trap and interfaced to an Agilent 7890A gas chromatograph equipped with a 5975 inert XL mass spectrometer. GC/MS operating parameters were selected to support EPA Method 8260 analysis.
Purge and Trap Parameters
- Concentrator: EST Encon Evolution
- Trap type: Vocarb 3000
- Valve oven temperature: 150 °C
- Transfer line temperature: 150 °C
- Trap temperature: 35 °C
- Moisture Reduction Trap (MoRT) temperature: 39 °C
- Purge time: 11 min
- Purge flow: 40 mL/min
- Dry purge Temp.: Ambient
- Dry Purge Flow: 40mL/min
- Dry Purge Time: 1.0 min
- Desorb pressure Control: ON
- Desorb Pressure: 12 psi
- Desorb time: 1.0 min
- Desorb temperature: 250 °C
- MoRT bake temperature: 150 °C
- Bake temperature: 265 °C
- Sparge vessel bake temperature: 130 °C
- Bake time: 8 min
- Bake flow: 40 mL/min
GC/MS Parameters
- System: Agilent 7890A/5975
- Inlet: Split/splitless mode, 200 °C, 40:1 split ratio, 17.311 psi inlet head pressure
- Column: Rtx-624 20m x 0.18mm I.D. 1µm film thickness
- Oven program: 45 °C (1 min) → ramp 18 °C/min → 220 °C, hold for 0.3 min
- Column flow rate: 0.8 mL/min helium
- Total flow: 38.8 mL/min
- Source temperature: 230 °C
- Quadrupole temperature: 150 °C
- Transfer line temperature: 180 °C
- Scan range: m/z 35–265, 3.12 scans/sec
- Solvent delay: 0.7 min
Standards and Calibration
Oxygenate standards were obtained from Restek. The California oxygenate mixture included:
- Diisopropyl ether (DIPE)
- Ethyl tert-butyl ether (ETBE)
- tert-Amyl methyl ether (TAME)
- tert-Butyl alcohol (TBA)
- Methyl tert-butyl ether (MTBE)
Most ether compounds were prepared at 2000 µg/mL, while TBA was prepared at 10,000 µg/mL. Ethanol standards were also obtained from Restek at 10,000 µg/mL.
Calibration curves consisted of nine concentration levels, ranging from 0.5 to 200 ppb for ether compounds and 2.5 to 1000 ppb for TBA and ethanol. Method detection limits were determined using seven replicate injections at low calibration levels.
Results and Discussion
Evaluation of purge volume and purge temperature demonstrated that increasing sample volume resulted in improved compound response. Additionally, the use of a heated purge at 60 °C significantly enhanced the response of polar compounds, particularly ethanol and TBA, with ethanol response more than doubling under heated purge conditions.
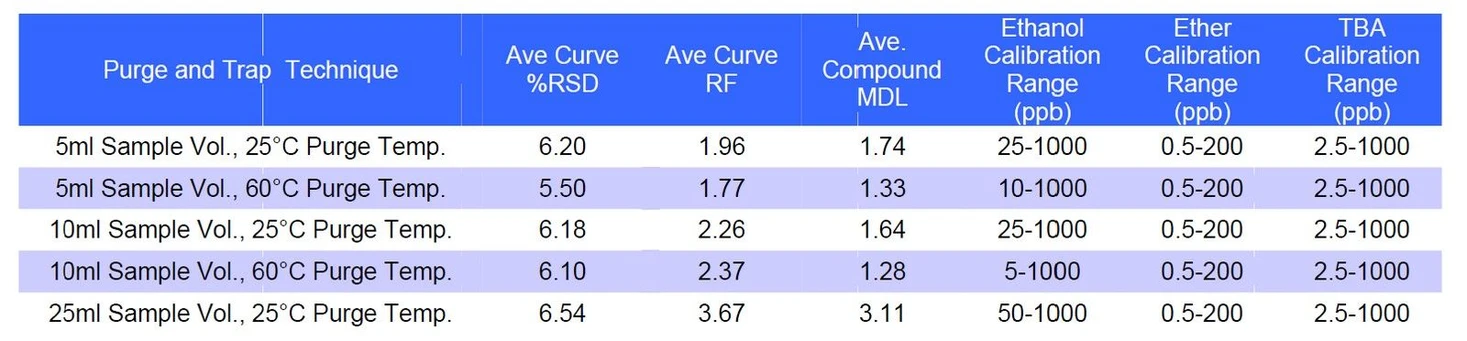 EST Analytical: Table 3: Sample Volume and Temperature Experimental Results Summary
EST Analytical: Table 3: Sample Volume and Temperature Experimental Results Summary
While larger sample volumes generally increased sensitivity, the 10 mL sample volume combined with a 60 °C purge temperature provided the best balance of sensitivity, linearity, and chromatographic performance. This condition also produced the most consistent linear calibration range, especially for ethanol, which is traditionally difficult to analyze using purge-and-trap techniques.
Chromatograms comparing room-temperature purge and heated purge conditions (Figures 3 and 4) clearly illustrate the improved peak response and signal stability achieved with heated purging.
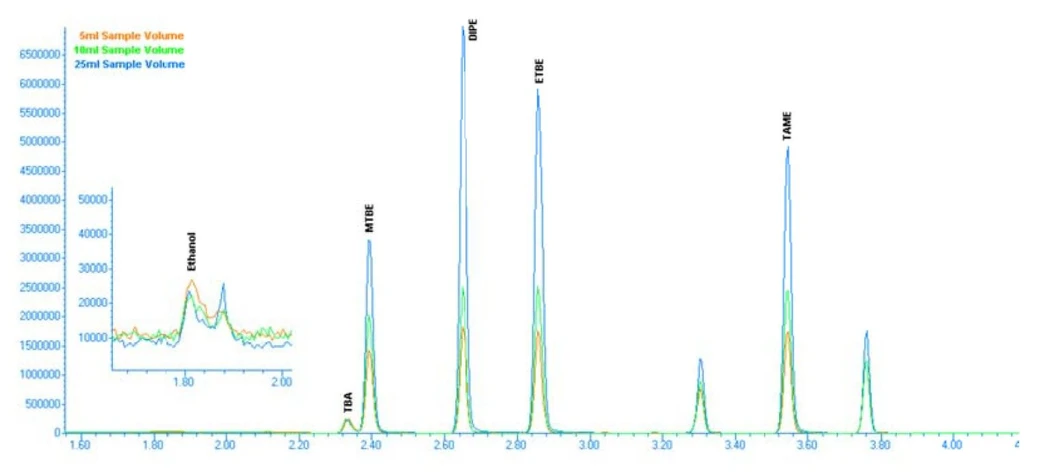 EST Analytical: Figure 3: 50ppb Chromatogram with Room Temperature Purge
EST Analytical: Figure 3: 50ppb Chromatogram with Room Temperature Purge
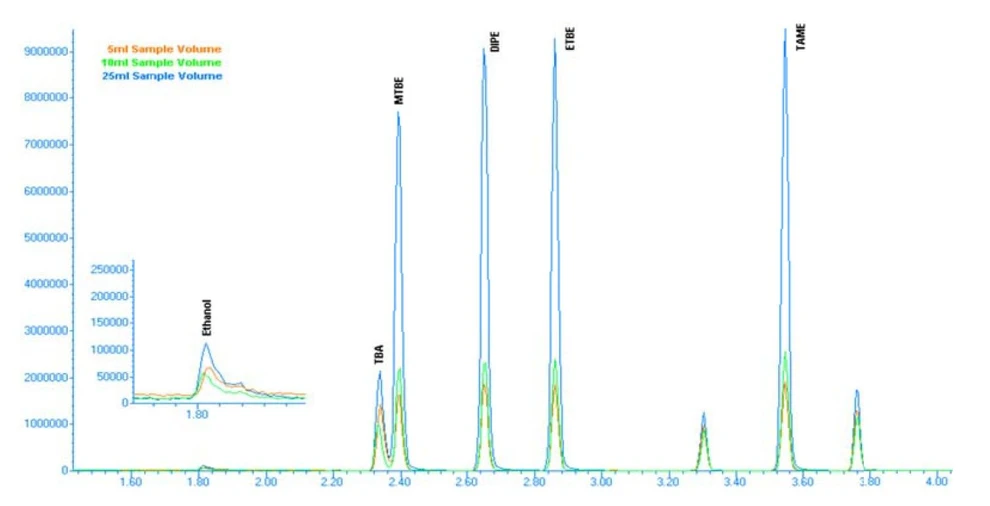 EST Analytical: Figure 4: 50ppb Chromatogram with 60 °C Purge
EST Analytical: Figure 4: 50ppb Chromatogram with 60 °C Purge
Full 8260 and California Oxygenate Analysis
Following optimization, a full EPA Method 8260 calibration and validation was performed using the selected conditions (10 mL sample volume, 60 °C purge). Method performance was evaluated for linearity, precision (%RSD), recovery, and detection limits across the complete compound list.
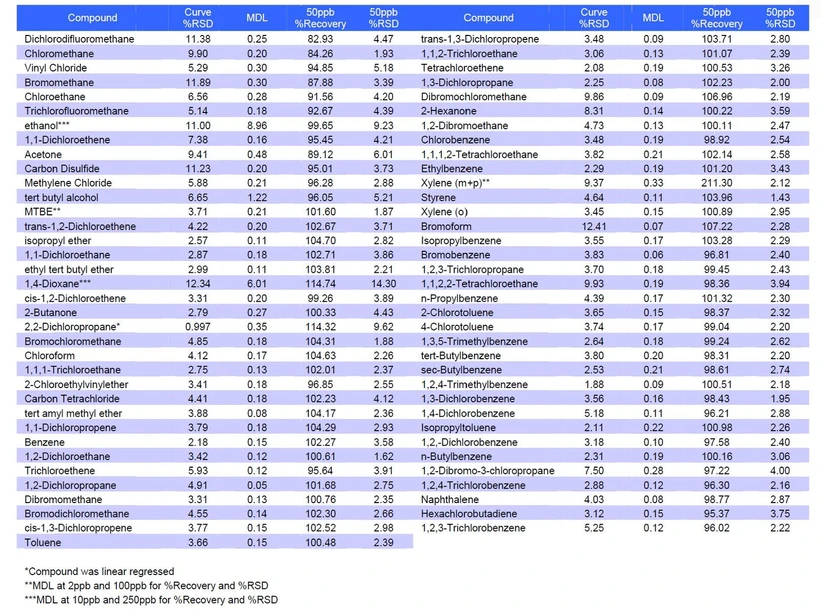 EST Analytical: Table 4: Summary of 8260 and California Oxygenate Data
EST Analytical: Table 4: Summary of 8260 and California Oxygenate Data
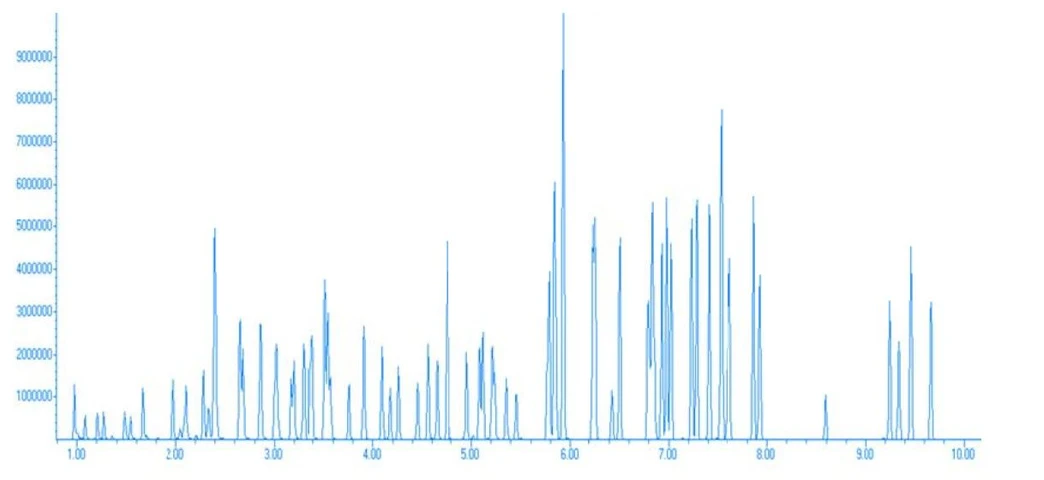 EST Analytical: Figure 5: 50ppb Standard of 8260 and Fuel Oxygenates
EST Analytical: Figure 5: 50ppb Standard of 8260 and Fuel Oxygenates
 EST Analytical: Figure 6: Extracted Ion Chromatogram of the 50 ppb Standard of the Fuel Oxygenate Compounds in the 8260 Mix
EST Analytical: Figure 6: Extracted Ion Chromatogram of the 50 ppb Standard of the Fuel Oxygenate Compounds in the 8260 Mix
Results demonstrated that the system met or exceeded all method performance criteria. Recoveries and precision were within acceptable limits for both volatile organic compounds and oxygenates, confirming suitability for regulatory compliance applications.
Conclusions
Overall, fuel oxygenate compounds exhibited higher responses with increased sample volume, while purge temperature had the greatest impact on polar analytes, particularly ethanol and TBA. The heated purge at 60 °C, combined with a 10 mL sample volume, produced optimal linearity and sensitivity.
The EST Encon Evolution purge-and-trap system, equipped with a Moisture Reduction Trap (MoRT), proved to be highly effective for fuel oxygenate analysis. The system demonstrated excellent moisture control, robust analyte transfer, and reliable performance for both California oxygenates and full EPA 8260 compound lists. In all cases, method performance met or exceeded regulatory requirements.