Enhancing the targeted and untargeted analysis of honey by vacuum-assisted SPME-GC × GC-MS. A green, practical, and highly informative approach
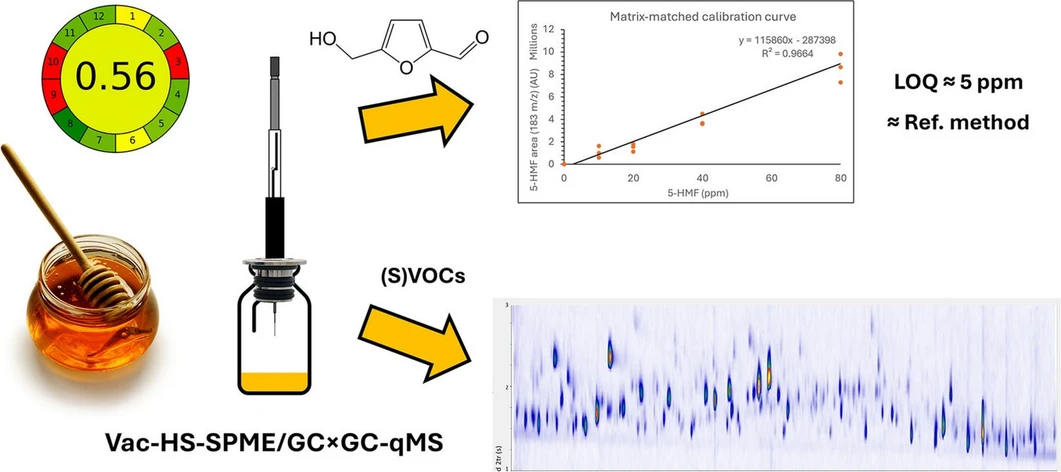
Green Analytical Chemistry, 12, 2025, 100207: Graphical abstract
Vacuum-assisted headspace solid-phase microextraction (Vac-HS-SPME) was coupled with comprehensive two-dimensional gas chromatography–mass spectrometry (GC×GC-MS) to enable sensitive and efficient analysis of honey. Reduced pressure accelerates mass transfer, allowing enhanced extraction of semi-volatile compounds at shorter times and lower temperatures.
The optimized Vac-HS-SPME approach enabled simultaneous quantification of 5-hydroxymethylfurfural (5-HMF) and comprehensive volatile fingerprinting for botanical and geographical authentication. Compared to conventional HS-SPME, signal intensities increased up to tenfold overall and up to 90-fold for less volatile compounds. The method achieved a validated LOQ of ~5 µg/g for 5-HMF and produced results comparable to the official HPLC-UV method, while offering a greener and more practical analytical workflow.
The original article
Enhancing the targeted and untargeted analysis of honey by vacuum-assisted SPME-GC × GC-MS. A green, practical, and highly informative approach
Damien Eggermont, Francesca Pardi, Giorgia Purcaro
Green Analytical Chemistry, 12, 2025, 100207
https://doi.org/10.1016/j.greeac.2025.100207
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Solid-phase microextraction (SPME) was introduced more than 30 years ago by Arthur and Pawliszyn as a solvent-free extraction technique [1]. Thanks to its simplicity and easiness in automation SPME, particularly in headspace mode (HS), has become a method of choice in several sectors such as environmental [2], clinical, pharmaceutical [3], and food sciences [4]. Over time, the traditional fiber-based technique has evolved, giving rise to new variants with different shapes and sorbent volumes, such as thin-film, SPME Arrow, or stir-bar sorptive extraction (SBSE) [5,6]. For HS mode, a dual equilibrium occurs between the sample and the HS, and between the HS and the sorbent phase [7]. One of the main challenges in HS-SPME is the ability to cover a broad range of volatility (particularly extending towards less volatile ones) and find the right compromise between sensitivity and extraction time. Indeed, compounds with low volatility can take an extremely long time to extract. Besides agitation to increase the mass transfer, the use of increased temperature can be beneficial but also can create artifacts, especially in matrices that can easily be altered by temperature, such as honey. An interesting alternative to enhance the extraction kinetics is using sub-ambient pressure, named vacuum-assisted (Vac-) HS-SPME. This technique was initially reported in 2001 by Brunton et al. for the analysis of meat volatile, where a 4- to 70-fold improvement in HS component extraction was observed for aldehydes and alcohols, compared to atmospheric pressure [8]. Starting in 2012, Vac-HS-SPME has been thoroughly theorized and explored [[9], [10], [11], [12], [13], [14], [15], [16]]. It has been highlighted that sampling below atmospheric pressure is particularly beneficial for compounds with a KH below 1.6 × 10–3 atm·m3/mol, while the more volatile compounds are not impacted. Vac-HS-SPME can thus provide a faster and more efficient extraction at milder temperatures, although a synergic effect with temperature was shown in the case of a highly viscous matrix as olive oil, as it reduces the viscosity favoring the mass transfer in the sample [17]. All in all, the use of Vac-HS-SPME allows the extraction of a much larger number of compounds, thus benefiting from the use of more powerful chromatographic techniques, such as two-dimensional gas chromatography (GC × GC) coupled with mass spectrometry (MS) [4]. Nevertheless, most of the applications using Vac-HS-SPME so far have mainly focused on target analysis to prove the quantitative gain in extraction yield, apart from a few exceptions [18,19].
In this work, we aim to synergize from the coupling of two of such powerful techniques, namely Vac-HS-SPME and GC × GC-MS, by simultaneously optimizing a target application while not losing the advantages of the comprehensive vision obtained by GC × GC-MS.
A particularly challenging matrix, i.e., honey, a highly viscous and thermally sensitive matrix, was selected. One of the main quality parameters for this matrix is indeed represented by the detection of 5-hydroxymethylfurfural (5-HMF), a process contaminant formed by dehydration or Maillard reaction from sucrose, glucose, and fructose during heat processing and prolonged storage [20]. Its formation rate is also linked to additional parameters, such as water activity, pH, amino acid content, or cations content. Interested readers can refer to the review of Lee et al. for more details on the subject [21]. The 5-HMF content is used as a quality marker to assess the freshness of honey. It is regulated by the European Union (2001/110/EC) and the Codex Alimentarius (CXS 12–1981), with a maximum limit of 40 mg/kg for most honey and a higher limit of 80 mg/kg for honey coming from the tropical region [22,23]. The EU has set a limit of 15 mg/kg for honey with low enzymatic content, such as citrus honey, as freshness is typically assessed by 5-HMF content and diastase activity. In the case of low-enzymatic honey, diastase activity is not a reliable marker, so a lower 5-HMF limit is necessary to confirm freshness [24,25]. Regarding analytical methods, the International Honey Commission recognizes two spectrophotometric (White and Winkler method) and one reversed-phase High-Performance Liquid Chromatography Diode Array Detector (HPLC-DAD) methods based on dilution of honey followed by clarification and stabilization of the 5-HMF extract [[26], [27], [28]].
On the other hand, the volatile profile of honey has been widely studied as it encrypts information related to the botanical and geographical origin but also related to storage, processing, and environmental or microbial contamination [29,30]. Nevertheless, few papers have explored the use of GC × GC to unravel the complexity of the volatile profile and exploit the chromatographic fingerprinting information [[31], [32], [33]].
2. Materials and methods
2.4. GC × GC-QMS analysis
All the GC analyses were performed on a GCMS-TQ8050 NX (Shimadzu, Japan) using an INSIGHT™ reverse fill-flush flow modulator (SepSolve Analytical Ltd., UK) equipped with a sample loop of 0.53 mm i.d. × 22.6 mm (loop volume of 50 μL).
An apolar BPX-5 ms 20 m × 0.18 mm i.d. × 0.18 μm film thickness (equivalent to 5 % phenyl / 95 % dimethyl polysiloxane) (Trajan, AU) column and a mid-polar SLB-50 ms 3 m × 0.25 mm i.d. × 0.25 µm film thickness (equivalent to 50 % phenyl / 50 % dimethyl polysiloxane) (Supelco, USA) were used as first and second column (1D, 2D), respectively. Two uncoated capillaries of 1 m × 0.1 mm with an auxiliary flow controller between them were used as auxiliary bleed lines.
The 1D and 2D helium flow were respectively 0.45 and 16 mL/min, whereas the equivalent bleed line flow was set at 0.5 mL/min. The modulation period was set at 2.5 s with a flushing time of 200 ms (kept constant during the entire run).
A passive T-splitter was used to reduce the flow reaching the MS detector, with ∼43 % (at the initial temperature) directed to the MS through an uncoated capillary of 1.1 m × 0.18 mm and the remainder discarded through a second uncoated capillary of 0.5 m × 0.2 mm.
The GC temperature was set at 35 °C for 1 min then heated up to 200 °C at 6 °C/min and to 280 °C (held 1 min) at 30 °C/min. The transfer line and ionization source temperatures were 250 °C and 200 °C, respectively. Simultaneous SIM and scan MS acquisition modes were used during the entire run, acquiring spectra from 45 to 350 m/z at 20 000 amu/s and SIM of 184 m/z. Each event had an acquisition time of 0.025 ms.
Data were acquired using GCMS solution v4.53 (Shimadzu, Japan) and were converted into AIA file to be imported and treated in ChromSpace software v2.1.7 (SepSolve Analytical Ltd., UK). NIST17 and FFNSC 3.0 MS libraries were used for identification.
3. Results and discussion
3.4. Volatile chromatographic fingerprint by Vac-HS-SPME-GC × GC
The advantages of using GC × GC for untargeted volatile analysis of food commodities are undoubtedly, and they are largely discussed in the literature [41,42]. Using GC × GC-MS allows for generating highly informative chromatographic fingerprints, which encrypt information related to multiple questions. In the case of honey, the volatilome may encrypt information related to botanical and geographical origin [33].
The goal of this work was to combine the advantages of chromatographic fingerprinting of GC × GC with the enhanced volatile profiling obtained in Vac-HS-SPME to optimize a simultaneous targeted (5-HMF) and untargeted approach for honey analysis. Nevertheless, the untargeted analysis discussed here is a proof-of-concept to show the potentiality, and no authenticity questions were explored since a dedicated sampling was not available at the moment of the study. Nevertheless, as VOCs' ability to answer this kind of questions is widely proven in the literature [19,29], it was reckoned important to discuss the general advantages of this approach in terms of enhanced volatile profile thanks to the combined application of Vac-HS-SPME and GC × GC-MS.
On the data acquired using the Dohelert design discussed for 5-HMF, a response surface for the total area of volatiles in HS-SPME and Vac-HS-SPME was also built (Fig. 3, and details in Figs. S5 and S6). As for the 5-HMF (Fig. 1), no equilibrium was reached during the extraction occurring at atmospheric pressure, whereas a clear time-optimum was reached for extraction under vacuum (around 20 min). Regarding the significance of parameters impacting the extraction, vacuum extraction shows a quadratic impact of the time and the temperature, a linear impact of the time, and a near to significant impact of the temperature. With regard to atmospheric extraction, although the second-order regression is significant, no parameter has a significant impact on extraction. This could be explained by a high collinearity between the impact of the parameters and was indeed confirmed by a high variance inflation factor (> 5). A stepwise variable selection was applied to reduce the number of variables. Time and temperature were selected, and a significant impact on the extraction was demonstrated, with a general trend similar to Fig. 4A (details about the restricted model can be found in Fig. S6 and Table S7).
 Green Analytical Chemistry, 12, 2025, 100207: Fig. 3. Response surface obtained considering the total area of volatile using HS-SPME (A) and Vac-HS-SPME (B). Extraction of 0.5 g of aged honey.
Green Analytical Chemistry, 12, 2025, 100207: Fig. 3. Response surface obtained considering the total area of volatile using HS-SPME (A) and Vac-HS-SPME (B). Extraction of 0.5 g of aged honey.
 Green Analytical Chemistry, 12, 2025, 100207: Fig. 4. Comparison of the volatile fraction extracted from 0.5 g of aged honey for 17 min at 60 °C at A) Vac-HS-SPME, B) HS-SPME, C) bubble plot representing the ratio Vac-HS-SPME/HS-SPME orange bubbles and HS-SPME/Vac-HS-SPME blue bubbles. The size of the bubble is proportionally bigger at the increase of the ratio, with the size of the ratio = 1 represented by the size of the bubbles in the legend.
Green Analytical Chemistry, 12, 2025, 100207: Fig. 4. Comparison of the volatile fraction extracted from 0.5 g of aged honey for 17 min at 60 °C at A) Vac-HS-SPME, B) HS-SPME, C) bubble plot representing the ratio Vac-HS-SPME/HS-SPME orange bubbles and HS-SPME/Vac-HS-SPME blue bubbles. The size of the bubble is proportionally bigger at the increase of the ratio, with the size of the ratio = 1 represented by the size of the bubbles in the legend.
As for 5-HMF, the total signal with Vac-HS-SPME was much higher (about 10-fold higher), especially for the less volatile, as also shown in Fig. 4, Fig. 5. The overall profile of the response surfaces was very similar to the ones obtained for the single 5-HMF, having almost the same optimal conditions of extraction, which means that while determining 5-HMF, information regarding the entire volatilome can also be maximized, increasing the applicability and the level of information that can be extracted from a single analysis, performing simultaneous targeted and untargeted analysis of honey.
 Green Analytical Chemistry, 12, 2025, 100207: Fig. 5. Expansion and MS identification of the three compounds zoomed in the box, which are partially coeluted in the first dimension. The MS spectra, along with the similarity match with the NIST library is reported, for the single peaks and the merged of the three.
Green Analytical Chemistry, 12, 2025, 100207: Fig. 5. Expansion and MS identification of the three compounds zoomed in the box, which are partially coeluted in the first dimension. The MS spectra, along with the similarity match with the NIST library is reported, for the single peaks and the merged of the three.
Fig. 4 shows the overall profile obtained using HS-SPME and Vac-HS-SPME, highlighting the position of 5-HMF in the middle of the chromatogram (∼15 min). Starting from the elution of 5-HMF the difference between the two chromatograms becomes more evident in intensity, with a clear enhanced extraction using Vac-HS-SPME. To better highlight the difference between HS-SPME and Vac-HS-SPME, all the compounds with a signal-to-noise ratio higher than 30 were integrated. The ratios of the areas were used to build the bubble plot corresponding to the chromatographic distribution of the compounds considered. When the HS-SPME area was higher than the Vac-HS-SPME, it was plotted in grey in Fig. 4C, while when the Vac-HS-SPME was higher than the HS-SPME, it was plotted in orange. The size of the bubble is proportional to the value of the ratio, with 1 (no difference between Vac- and HS-SPME) corresponding roughly to the size of the bubble in the legend (Fig. 4C). It is worth noticing that in the early eluted compounds (up to about 12 min), generally, more grey spots are found, which correspond to compounds better extracted with HS-SPME. However, as a reminder, ratios similar to 1 means no significant discrepancy between Vac- and HS-SPME. In this regard, it is known from the theory that most volatile compounds (more precisely, compounds with KH > 1.6 × 10–4) are not impacted by the use of reduced pressure conditions [43], and this is confirmed by the small size of the grey spot, indicating a slightly higher signal obtained by HS-SPME compared by Vac-HS-SPME. Indeed, out of the 24 compounds eluted in this area, 18 were more intense using HS-SPME than Vac-HS-SPME, and out of these 18, only four compounds showed a ratio (HS/Vac) > 2 (max 2.6), four between 1.6 and 1.9, 5 between 1.3 and 1.5, and five between 1 and 1.2.
Despite the minimal difference in this area, a loss of the most volatile compounds during the evacuation step, performed to reduce the pressure in the vial, cannot be excluded entirely, despite this step was performed in frozen samples and keeping the sample in ice during the evacuation.
Nevertheless, the gain of signal in the later part of the chromatogram (from about 14 min) is outstanding, having values up to 90 times more intense using Vac-HS-SPME compared to HS-SPME.
The complexity of the volatile profile of honey significantly benefits from the increased separation power obtained using GC × GC-MS, allowing for a better definition of chromatographic fingerprinting and simplifying the identification. An example of the improved separation is reported in the chromatographic expansion reported in Fig. 5. It is highlighted as the identification, even of a major peak as benzoic acid (in its trimethylsilyl -TMS- form), is improved (from 80% to 94% of MS similarity match) when well separated from the coeluted glycerol and octanoic acid TMS derivatives, as well as the other way around.
4. Conclusion
Vac-HS-SPME was investigated as an alternative technique for accurately determining 5-HMF in honey. The proposed method was validated, meeting the limits imposed by EU legislation. A comparison with the established HPLC-DAD method showed similar results, confirming its fit-to-purpose. The proposed method was greener than the reference method while at the same time providing an additional information-rich chromatographic fingerprint of the volatilome, which can be used for botanical and geographical authenticity studies. The use of Vac-HS-SPME significantly increased the extraction of the less volatile compounds, and the richer profile benefited from using advanced chromatographic techniques, such as GC × GC, for a more robust characterization.
In terms of practicality, the proposed method performed similarly to the reference method, but the former allowed for simultaneous targeted and untargeted analysis in a single determination, making it an interesting approach to answer multiple scientific questions in a single study.




